- Cart 0
- English
Absin ELISA Powers Montmorillonite Oral Vaccine: Mucosal Immunity Unlocked for Colorectal Cancer
January 16, 2026
Clicks:111
Against the backdrop of an ever-increasing global cancer burden, colorectal carcinoma—one of the most prevalent mucosal neoplasms—remains notoriously recalcitrant to conventional vaccines that fail to elicit mucosal immunity. Recently, a breakthrough published in the Journal of the American Chemical Society (J. Am. Chem. Soc.) describes an orally administered therapeutic vaccine (Mn-MMT@OVA) constructed from Mn2+-enriched montmorillonite nanosheets, opening a new avenue for immunotherapy of colorectal cancer. Absin ELISA kits empowered this study and witnessed the milestone achievement in mucosal-immune-activation strategy.
Title: Montmorillonite-Based Oral Vaccine for Colorectal Cancer Immunotherapy through Mucosal Immune Activation
Journal: Journal of the American Chemical Society (IF 15.6) | DOI: https://doi.org/10.1021/jacs.5c06776
Absin products used: Mouse IL-4 ELISA Kit (abs520003), Mouse IL-10 ELISA Kit (abs520005), Human/Mouse/Rat TGF-β1 ELISA Kit (abs552208)

I. Background: The “Pain Points” of Mucosal-Tumor Therapy and the “Opportunity” of Oral Vaccines
Mucosal tumors such as colorectal carcinoma originate in mucosal tissues and constitute a major fraction of the global cancer burden. The mucosal immune system harbours ~80 % of the body’s immune cells and is the frontline defence against mucosal neoplasia. However, conventional sub-cutaneous or intramuscular vaccines suffer from “immune compartmentalization,” rendering them unable to prime mucosal immunity and leading to poor clinical efficacy.
Oral vaccines can exploit the intestine—the largest mucosal immune organ—and offer superior safety and patient compliance, yet they face three major hurdles: (i) antigen degradation by gastric acid and digestive enzymes, (ii) antigen dilution across the vast intestinal surface, and (iii) suppression of immune responses by oral tolerance. Overcoming these bottlenecks is the central challenge in developing oral tumour vaccines.
II. Design Rationale: Precision Engineering for “On-site” Vaccine Activity in the Gut
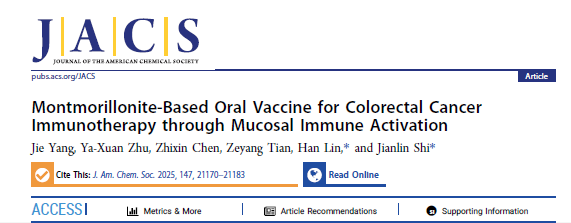
The investigators innovatively selected montmorillonite (MMT) as a carrier and, via ion-exchange, intercalated Mn2+ and the tumour antigen ovalbumin (OVA) into its interlayer galleries, yielding the oral vaccine Mn-MMT@OVA. The design logic is as follows:
1. Carrier choice: MMT is naturally resistant to the harsh gastrointestinal milieu and exhibits strong mucoadhesion, serving as an “antigen depot” that protects OVA from degradation and enriches the formulation at the intestinal mucosa.
2. Immune activation: Released Mn2+ is a potent activator of the cGAS–STING axis, breaking oral tolerance by driving dendritic-cell (DC) maturation and antigen presentation.
3. Mucosal homing: Vaccine-primed lymphocytes acquire a “mucosal imprint,” expressing α4β7 and CCR9 homing receptors that guide their migration to colonic mucosal tumour sites for targeted cytotoxicity.
III. Key Results: Multidimensional Validation of Antitumour Potential
1. Robust nano-architecture and mucosal enrichment
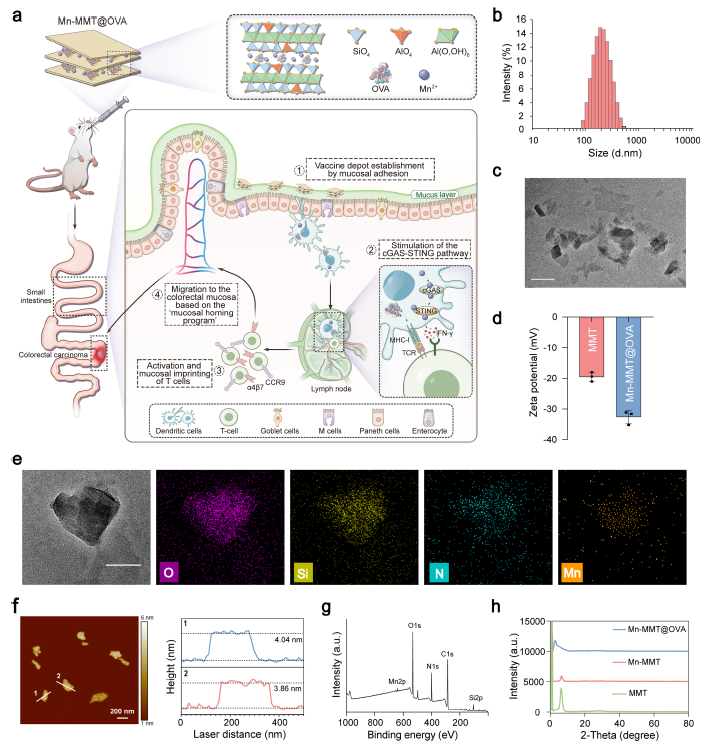
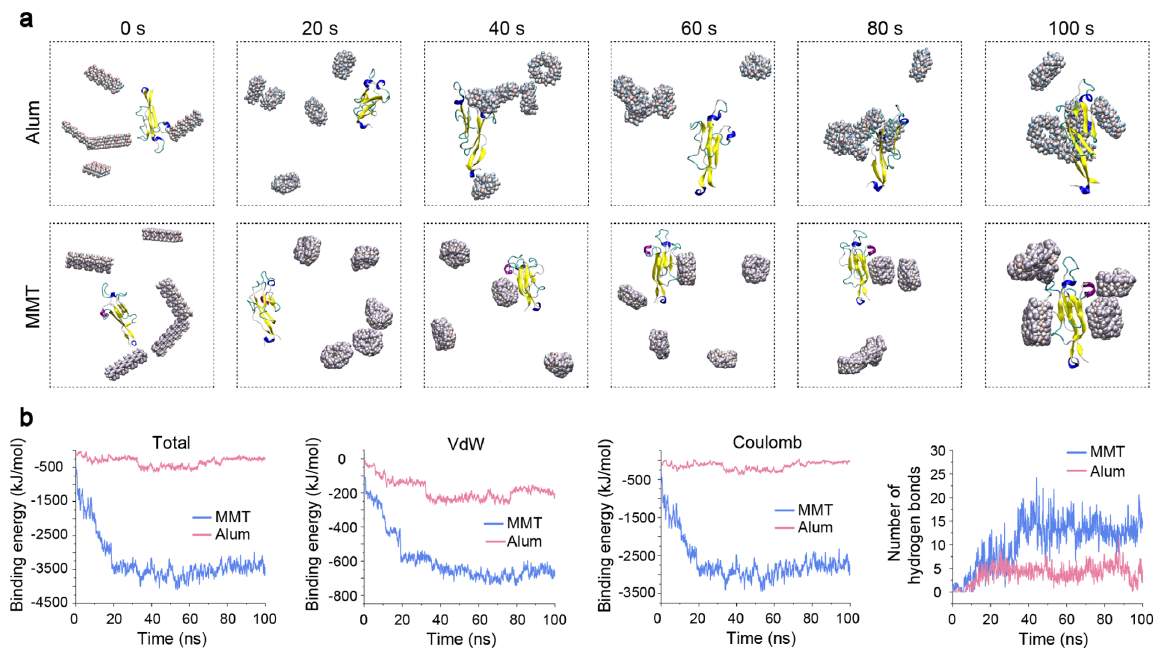
TEM and AFM confirmed the typical lamellar architecture of Mn-MMT@OVA (mean size 182 nm; interlayer spacing expanded to 3.36 nm), stably accommodating OVA (64.2 wt % loading) and Mn2+ (7.15 wt %). In simulated gastric and intestinal fluids the formulation retained structural integrity, releasing only ~20 % of its cargo within 2 h and reaching 80 % cumulative release after 48 h in the intestinal phase, thereby avoiding gastric “burst release” (Fig. 1 & 2 in the original paper).
Original Fig. 1: Synthesis and characterisation of Mn-MMT@OVA
Original Fig. 2: Mucoadhesion and depot formation of Mn-MMT@OVA
Via hydrogen bonding, van-der-Waals and electrostatic interactions with mucin-2 (MUC2), Mn-MMT@OVA established a “vaccine depot” that doubled intestinal residence time compared with an Alum-adjuvanted control (Fig. 2e–g & 3).
Original Fig. 3: Molecular-dynamics simulation of MUC2–vaccine interaction
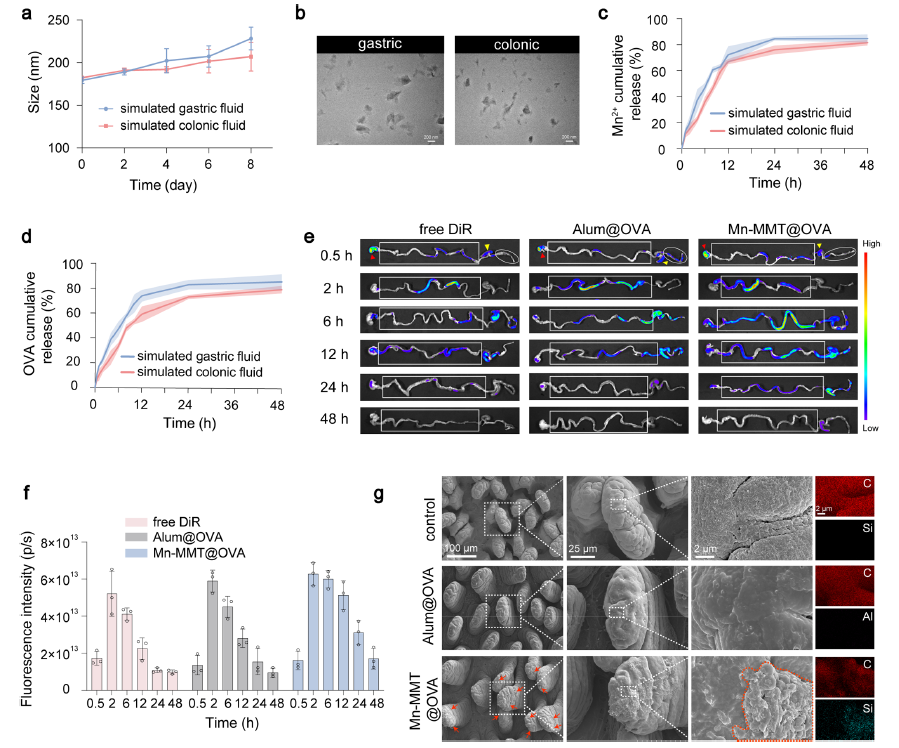
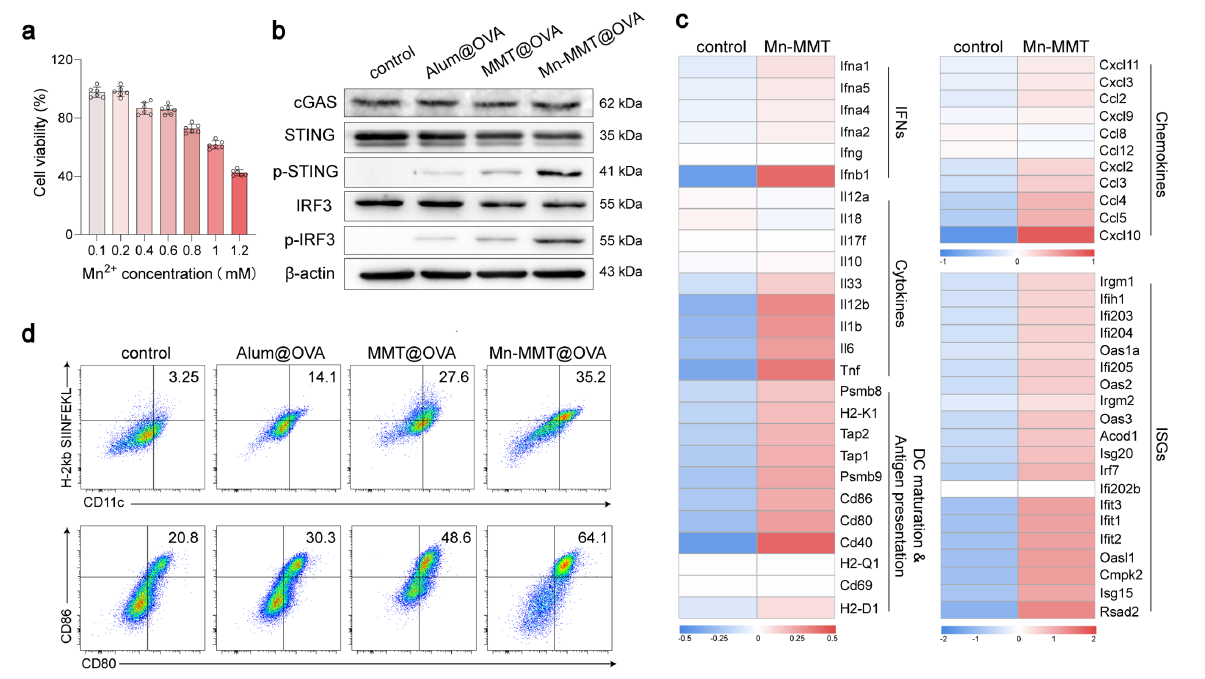
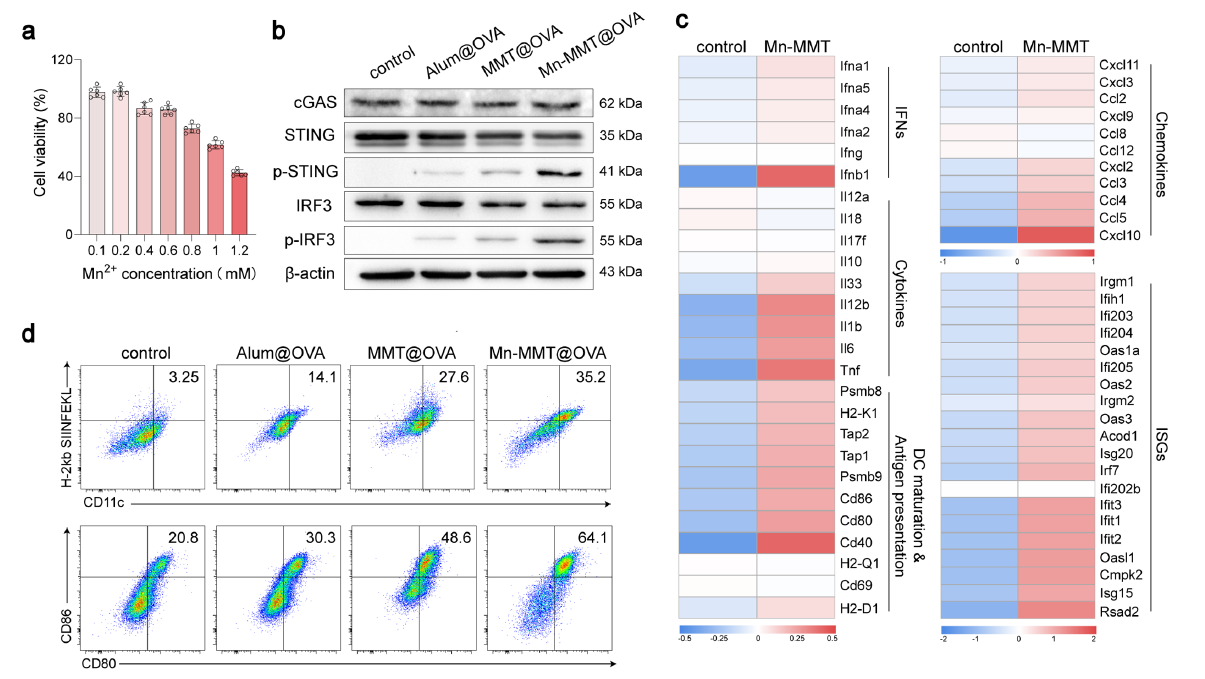
2. Potent cGAS–STING activation breaks immune tolerance
Mn2+ released from Mn-MMT@OVA robustly activated the cGAS–STING pathway in DCs, elevating phosphorylated STING (p-STING) and IRF3 (p-IRF3), which promoted DC maturation (64.1 % CD11c+/CD80+/CD86+) and antigen presentation (86.0 % CD11c+/H-2Kb-SIINFEKL+), markedly outperforming Alum (Fig. 4a–d).
Original Fig. 4: cGAS–STING activation by Mn-MMT@OVA
3. Re-programming of the gut immune micro-environment
The vaccine significantly reduced intestinal IL-10 and TGF-β, increased CD8+ T-cell infiltration, and skewed macrophages toward an MHC-II+ pro-inflammatory phenotype. CyTOF revealed pronounced enrichment of T, B and DC subsets, laying the groundwork for systemic antitumour immunity (Fig. 5).
Original Fig. 5: Modulation of the gut immune micro-environment
4. Profound tumour growth inhibition with concomitant mucosal and systemic immunity
In an orthotopic colorectal-cancer model, oral Mn-MMT@OVA suppressed tumour growth by >70 % versus saline controls within 20 days, with extensive necrotic areas evident histologically (Fig. 6). Simultaneously, the vaccine elicited high levels of anti-OVA IgA in the intestinal lumen (mucosal immunity) and IgG in serum (systemic immunity), while promoting infiltration of CD3+/α4β7+ and CD3+/CCR9+ homing T cells into tumours, where CD8+ T cells reached 8.03 % of total cells (Fig. 7).

Original Fig. 6: In-vivo antitumour efficacy
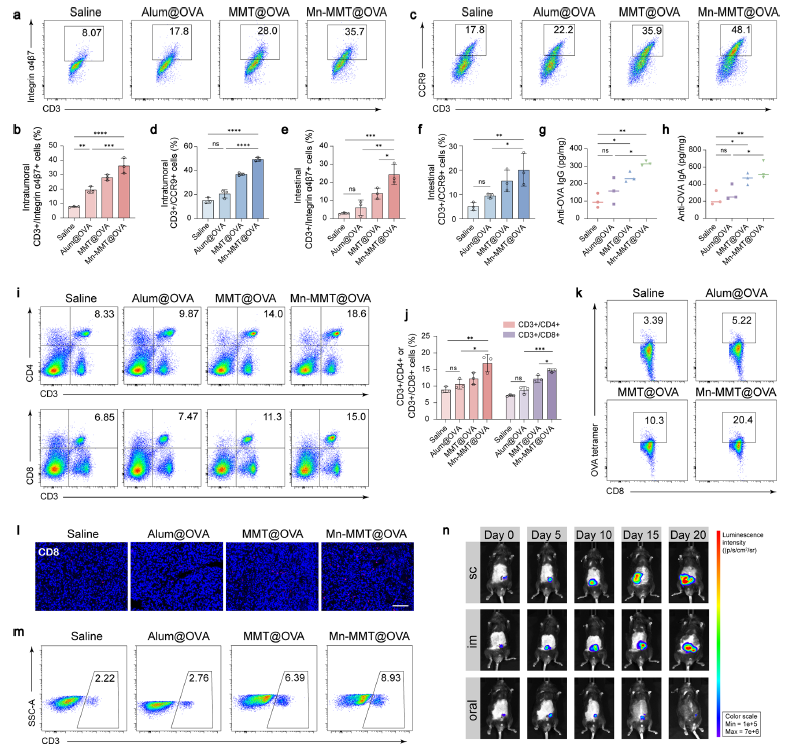
Original Fig. 7: Induction of mucosal and systemic immune responses
5. Excellent biocompatibility without overt toxicity
Mice maintained stable body weight, and no morphological abnormalities were observed in heart, liver, spleen, lung or kidney; haematological and serum biochemical indices remained within normal ranges, confirming an acceptable safety profile (Fig. 6f & Figs. S16–S17).
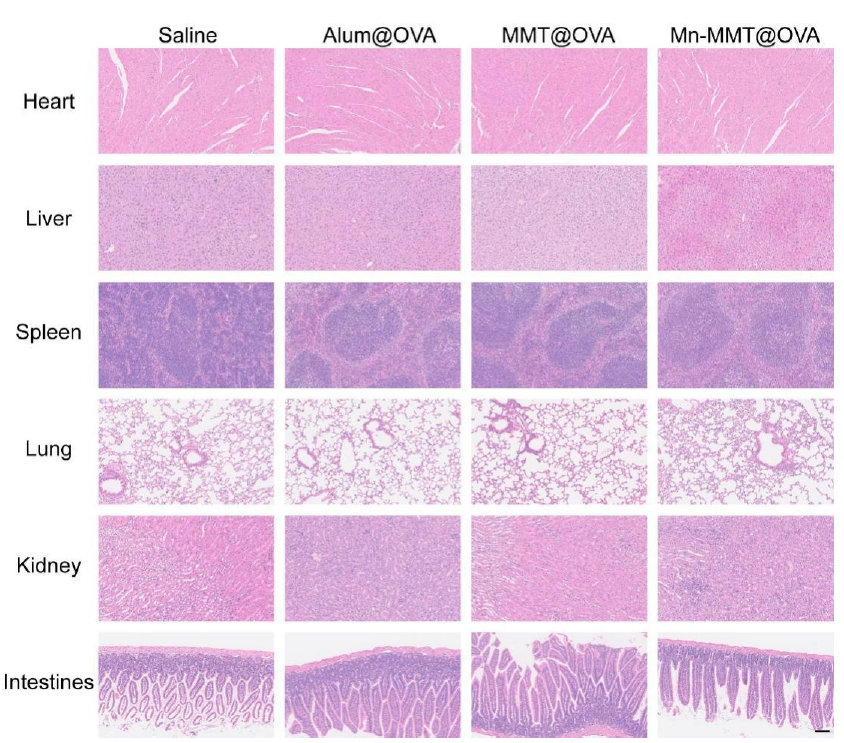
Original Fig. S16: Histological analysis of major organs
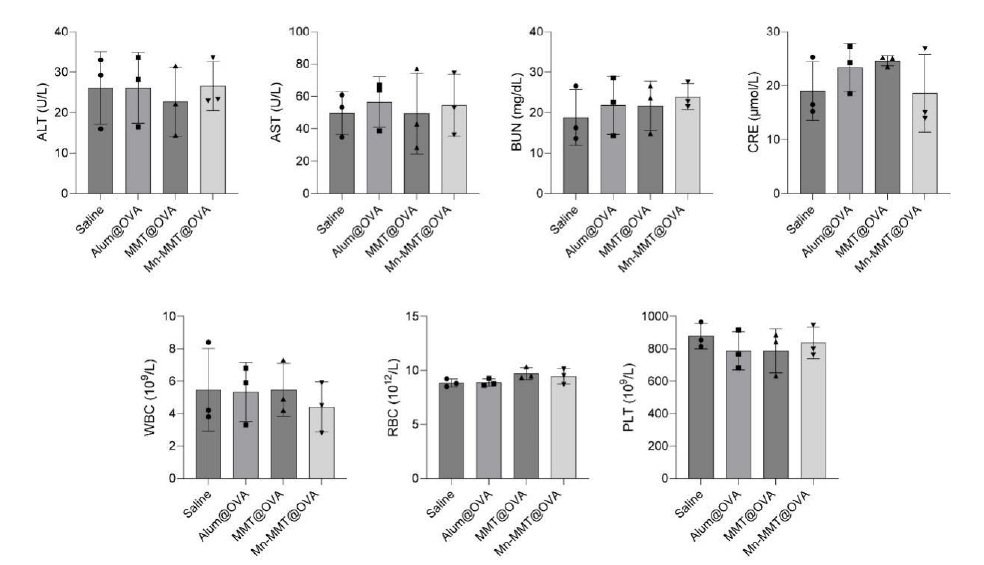
Original Fig. S17: Haematological and serum biochemical parameters
IV. Powered by Absin: Critical-tool Support for Scientific Breakthroughs
In this high-impact study, Absin’s flagship kits provided reliable quantification of intestinal immune mediators:
Mouse IL-4 ELISA Kit (abs520003): Specific quantification of IL-4 in murine intestinal tissues, clarifying vaccine-induced modulation of Th2 immunity and micro-environmental homeostasis.
Mouse IL-10 ELISA Kit (abs520005): Accurate determination of intestinal IL-10, validating the vaccine’s ability to dampen immunosuppressive cytokines and break oral tolerance (Fig. 5d).
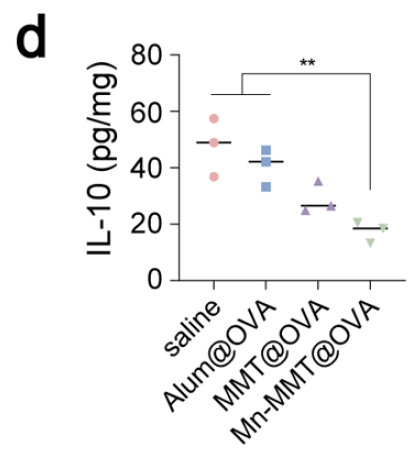
Original Fig. 5d: Intestinal IL-10 quantification with Absin Mouse IL-10 ELISA Kit
Human/Mouse/Rat TGF-β1 ELISA Kit (abs552208): Cross-species, high-sensitivity detection of TGF-β1, providing key evidence that the vaccine down-regulates immunosuppressive cytokines and reshapes the gut immune micro-environment (Fig. 5e).
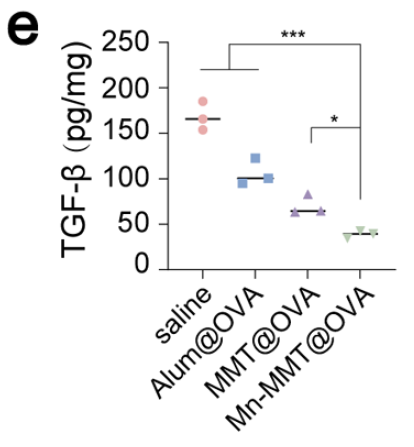
Original Fig. 5e: Intestinal TGF-β1 quantification with Absin Human/Mouse/Rat TGF-β1 ELISA Kit
V. Conclusions & Perspectives
This study innovatively integrates the mucoadhesive properties of montmorillonite with the immunostimulatory activity of Mn2+, creating the first montmorillonite-based oral vaccine for colorectal cancer and overcoming the long-standing bottleneck of mucosal immune activation. As a dedicated reagent supplier, Absin continues to provide high-quality tools that empower cutting-edge research in tumour immunotherapy, nano-drug delivery and beyond.
This article is based on the original publication in the Journal of the American Chemical Society (DOI: 10.1021/jacs.5c06776). All figures and data cited remain the intellectual property of the original journal and the authoring research team. Should any infringement occur, please contact us for prompt removal; we will cooperate fully.
|
Cat. # |
Product |
Size |
| Mouse IL-4 ELISA Kit | 96T | |
| abs520005 | Mouse IL-10 ELISA Kit | 96T |
Contact Absin
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
| Absin Bioscience Inc. worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
