- Cart 0
- English
WB Internal Control Selection Guide
1. What is internal control?
Internal controls are a class of proteins used in Western blot experiments to normalize and correct for experimental errors. They are usually relatively constant proteins expressed in different tissues and cells, encoded by housekeeping genes, and are often used as a reference for detecting changes in the expression level of the protein of interest.
2. What is the role of internal control in WB?
Correction of experimental error: The internal control is used to correct the experimental error in the process of protein quantification and sample loading to ensure the accuracy of the experimental results. Since there may be operational errors in protein extraction, quantitation, loading, etc., different samples can be corrected by using internal controls.
Semi-quantitative analysis: Experimental results can be semi-quantitatively analyzed by comparing the signal intensity of the internal control and the target protein. If the amount of protein in the sample is limited, it is only enough to perform an electrophoresis transfer experiment, the internal parameter and the amount of target protein of the sample are measured respectively, and then the amount of the target protein of each sample is divided by its internal control content respectively, and the relative content of the target protein in each sample after internal control correction is obtained, and this value is used for comparison and analysis between samples.
As a blank control: The internal control can be used as a blank control to detect whether the protein transfer is complete and whether the entire Western blot color development or luminescence system is normal.
Ensure comparability of experimental results: When comparing the relative expression of the protein of interest under different conditions or in different tissues, the internal control provides a reference standard to ensure the comparability of experimental results.
3. What are the common WB internal references?
β-Actin: β-Actin is a cytoskeletal protein with a molecular weight of about 42KDa, which is widely distributed in various tissues and is abundantly expressed and relatively constant. It is commonly used as an internal control for total protein, especially in non-muscle tissues. However, it is important to note that β-Actin may be expressed at lower levels in some specific tissues (e.g., myocardium) and therefore may not be applicable in all cases. In addition, the distribution of different β-Actin isoforms in different tissues varies, which should be taken into account when selecting antibodies.

Rat brain lysate 20 μg

Human colon cancer tissue

Hela cells
abs171598 Rabbit anti-β-Actin Monoclonal Antibody
GAPDH (glyceraldehyde-3-phosphate dehydrogenase): Around 36 kD, GAPDH is a widely expressed protein that is suitable for a wide range of cell types. However, under certain conditions, such as hypoxia or diabetes, the expression of GAPDH may be affected, so careful selection is required depending on the experimental conditions.
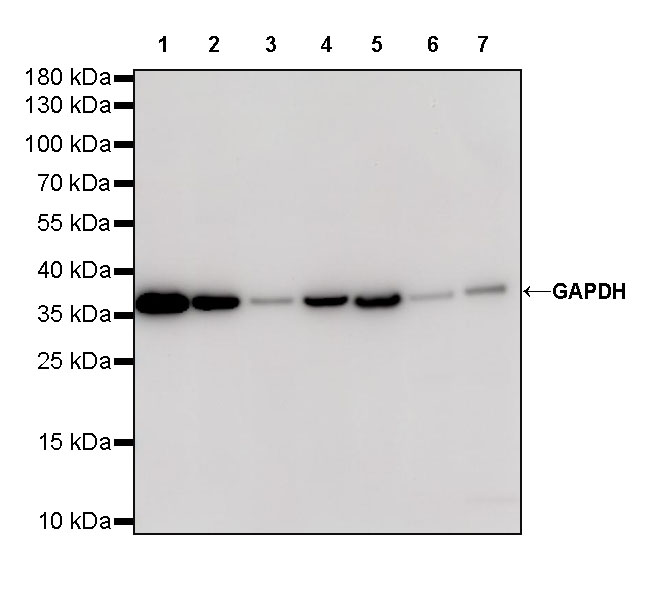
abs132004 Rabbit anti-GAPDH Polyclonal Antibody
β-Tubulin: about 55 kDa, β-Tubulin is a type of tubulin that is involved in the formation of the cytoskeleton. Due to its stable expression in cells, it is especially used for the detection of cytoskeletal proteins. The expression of β-Tubulin is relatively constant in a variety of cell types, but it is also necessary to consider expression variations under specific conditions, such as in apoptotic cells.

Hela cell whole lysate

Rat kidney tissue

NIH3T3 cells
abs171597 Rabbit anti-β-Tubulin Monoclonal Antibody
In addition to the internal control introduced above, there are also α-Tubulin, Hsp90, etc., the molecular weight and application of each internal reference are different, it is recommended to do your homework before choosing.
4. How to choose the right internal reference and what factors need to be considered?
Molecular weight of the protein of interest: The molecular weight of the reference protein should differ from the molecular weight of the target protein by at least 5 kDa to ensure that the bands of the target protein and the control can be clearly distinguished in gel electrophoresis and Western blot.
Sample species source: Samples from different species may require different reference proteins. For example, mammalian tissue or cell samples often choose β-actin, β-tubulin, GAPDH, etc., while plant samples may choose plant actin, Rubisco, etc. as internal controls.
Cell localization: Select the corresponding internal control based on the subcellular localization of the target protein. For example, if you are testing whole cell or cytoplasmic proteins, you can choose β-actin, β-tubulin, GAPDH, etc.; If it is a nuclear protein, Lamin B, Histone H3, etc. may be selected as internal controls.
Experimental environment: The selection of internal controls also needs to consider the actual experimental environment and the pretreatment of the sample. Under some special conditions, the expression of some reference proteins may change, which affects their stability and reliability as internal controls. For example, the expression of GAPDH may increase under conditions such as hypoxia or diabetes and may therefore not be suitable as an internal control.
Tissue specificity: Different tissues may require different internal controls. For example, a specific actin isoform may need to be selected as an internal control in muscle tissue, while β-actin may be selected in non-muscle tissue.
5. How to incubate the internal reference antibody and the target antibody?
Sequential incubation: After detecting the protein of interest, the antibody on the membrane is washed off using strip buffer, and then the antibody incubation and chromogenic detection of the reference protein are re-performed. This method requires the use of the same membrane during two incubations.
Double-membrane method: After protein transfer, the membrane is cut into two parts, large and small molecular weight, according to the size of the prestained protein Marker, so that the reference protein is separated from the target protein. The two membranes were then incubated and colored with an antibody to the reference protein and an antibody to the protein of interest, respectively.
Simultaneous incubation (if there is a large difference in molecular weight): If the molecular weight of the target protein is significantly different from that of the reference protein, both antibodies can be incubated on the same membrane after transfer. Due to the difference in band position, the control and target proteins can be detected separately on the same membrane.
Use of labeled reference antibodies: Some laboratories use pre-labeled control antibodies with an enzyme (e.g., HRP), which can be added during the secondary antibody incubation and developed as normal.
Recommended products
|
Catalog number |
Product name |
specification |
|
Rabbit anti-β-Actin Monoclonal Antibody |
100uL |
|
|
Rabbit anti-GAPDH Polyclonal Antibody |
100ug |
|
|
Rabbit anti-β-Tubulin Monoclonal Antibody |
100uL |
|
|
Mouse anti-β-Actin Monoclonal Antibody |
100uL |
|
|
Mouse anti-GAPDH Monoclonal Antibody |
100uL |
|
|
Rabbit anti-α-Tubulin Polyclonal Antibody |
100ug |
|
|
More...... |
||
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. Email: worldwide@absin.net |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
July 10, 2025
Clicks:151
