- Cart 0
- English
SUMO2-K11 Lactylation as a Therapeutic Target in Lung Adenocarcinoma: Absin Matrigel Enables Discovery of Ferroptosis Resistance Mechanism
February 09, 2026
Clicks:77
Lung adenocarcinoma (LUAD), the predominant subtype of non-small cell lung cancer (NSCLC), exhibits a 5-year survival rate of less than 26%, with urgent challenges in overcoming therapeutic resistance. Recently, a landmark study published in Cell Discovery unveiled a novel mechanism whereby SUMO2-K11 lactylation mediates ferroptosis resistance in LUAD through the regulation of ACSL4 degradation. Furthermore, the research team developed a cell-penetrating peptide (CPP) targeting this specific site, offering a promising new therapeutic strategy. In this groundbreaking study, Absin's Matrigel product served as a critical experimental reagent, providing essential support for the establishment of patient-derived organoid (PDO) models and facilitating the transition from mechanistic validation to clinical translation.
Publication Title: Ferroptosis-induced SUMO2 lactylation counteracts ferroptosis by enhancing ACSL4 degradation in lung adenocarcinoma
Journal: Cell Discovery (IF=12.5)
DOI: https://doi.org/10.1038/s41421-025-00829-6
Absin Product Used: Matrigel (High Concentration, Phenol Red-Free) (abs9493)
I. Research Background: The "Crosstalk" Between Lactylation and Ferroptosis—A Novel Therapeutic Target for LUAD
Tumor metabolic reprogramming and the regulation of cell death mechanisms represent central themes in cancer biology. It is established that:
1. LUAD cells preferentially utilize glycolysis for energy production even under aerobic conditions (aerobic glycolysis/Warburg effect), resulting in substantial lactate (LA) accumulation. This not only promotes tumor progression but also modulates protein function through lysine lactylation (Kla) post-translational modifications;
2. Ferroptosis is an iron-dependent form of regulated cell death driven by lipid peroxidation. While inducing ferroptosis can overcome resistance to chemotherapy and immunotherapy, the metabolic adaptations employed by LUAD cells to evade ferroptosis remain poorly understood.
The research team hypothesized that ferroptosis stress may regulate lactate metabolism and lactylation modifications, thereby establishing a "resistance feedback loop." This conjecture served as the central premise for the entire investigation.
II. Research Strategy: From Metabolic Screening to Clinical Translation—A Layered Approach to Deciphering Resistance Mechanisms
The team employed a classic research paradigm of "metabolic screening → modification identification → mechanistic validation → drug development → clinical model verification," systematically dissecting the ferroptosis resistance mechanisms in LUAD through four sequential steps:
Step 1: Metabolomic and Library Screening Identifies Lactate as the Critical Metabolite in Ferroptosis Resistance
Experimental Design: A549 and PC9 LUAD cells were subjected to ferroptosis induction using RSL3, IKE, and cisplatin (CDDP). An 889-compound endogenous metabolite library screen (|log₂(relative viability)| > 0.2) combined with untargeted metabolomics (|log₂(FC)| > 0.2, -log₁₀(p) < 1.301) was performed to identify key metabolites regulating ferroptosis sensitivity;
Key Findings: Lactate (LA) and sarcosine were identified as core regulators. LA levels were significantly elevated following ferroptosis induction and suppressed ferroptosis through dual mechanisms involving the lactate anion and acidic pH (e.g., reducing lipid peroxidation and increasing GSH/GSSG ratios) (Fig. 1d-j);

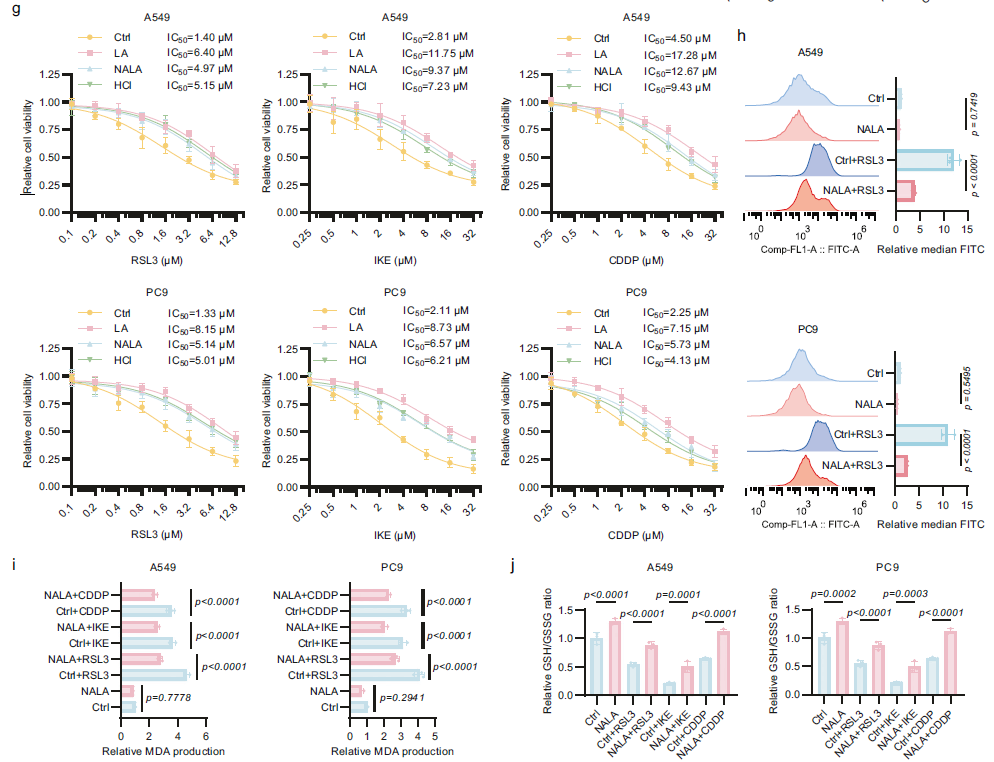
Mechanistic Insight: Ferroptosis induces mitochondrial damage, prompting cells to compensatorily enhance glycolysis, which further promotes LA accumulation. This establishes a preliminary cycle of "ferroptosis → glycolysis → LA elevation → ferroptosis resistance."
Step 2: Lactylation Modification Screening Identifies SUMO2-K11la as the "Master Switch" for Ferroptosis Resistance
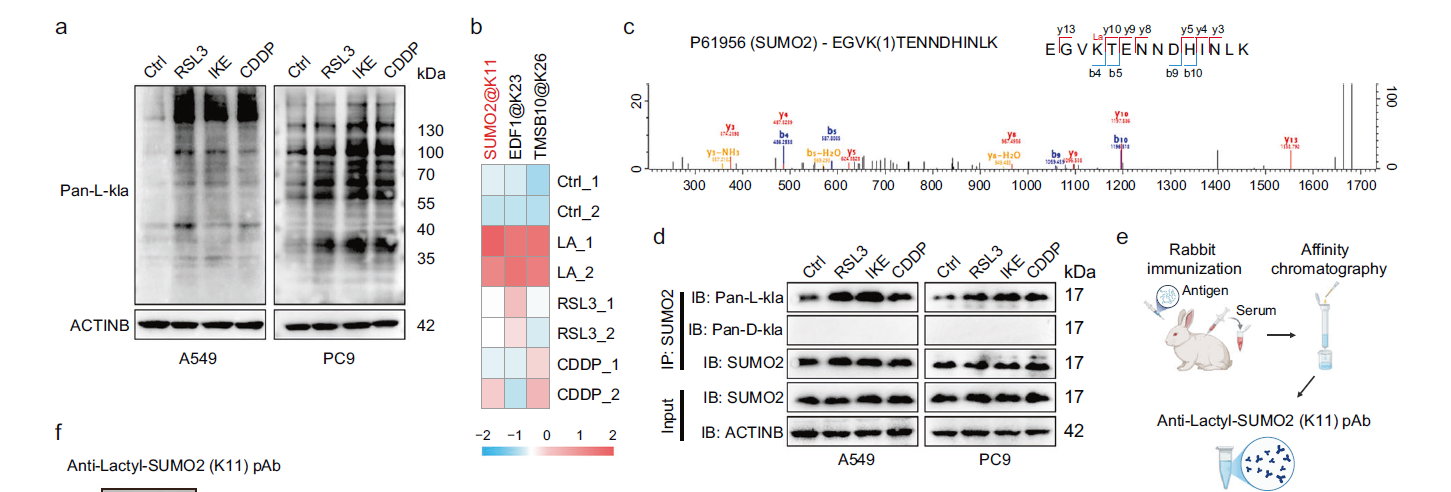
Experimental Design: Pan-lactyllysine antibody (pan-L-kla) enrichment coupled with mass spectrometry analysis was employed to screen for differentially abundant lactylation sites following ferroptosis induction;
Key Findings: SUMO2 protein lactylation at lysine 11 (SUMO2-K11la) was identified as the most significantly upregulated modification (Fig. 2a-c). CRISPR/Cas9-mediated generation of the SUMO2-K11R mutant (lactylation-deficient) further confirmed:
a. K11R mutant cells exhibited significantly enhanced sensitivity to ferroptosis (IC₅₀ reduced by >50%), and sodium lactate (NALA) could no longer induce resistance (Fig. 3a-c);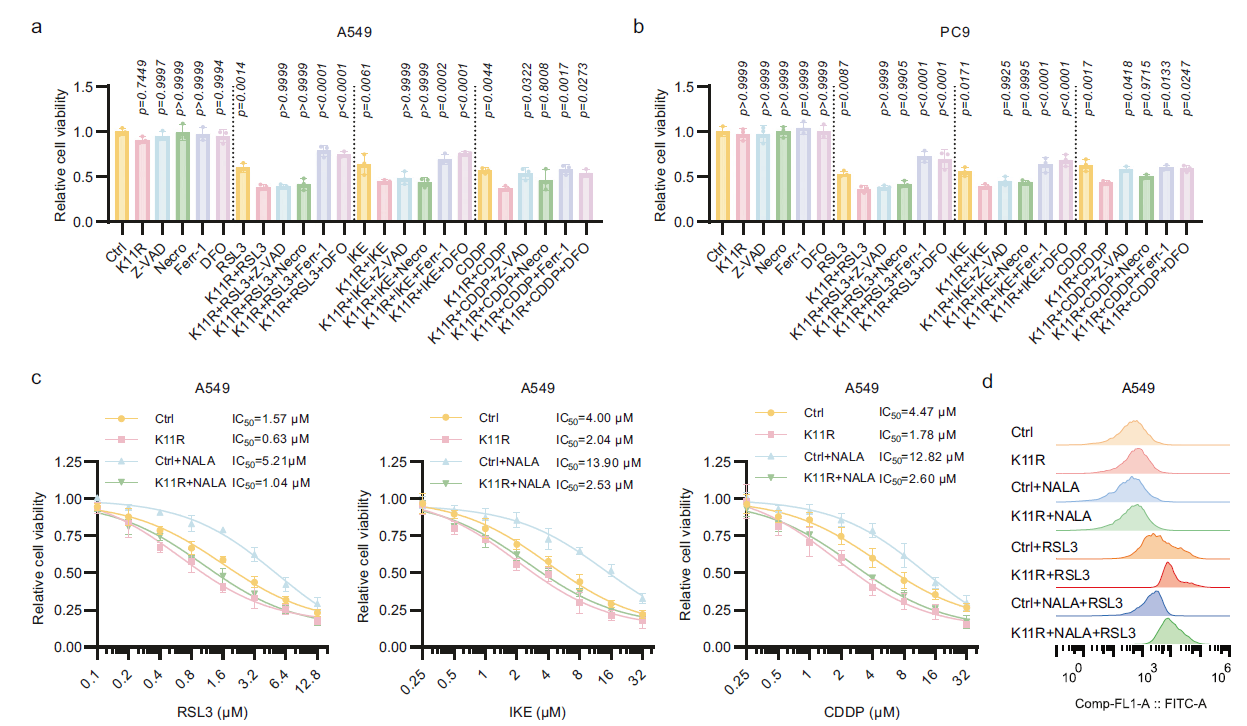
b. Clinical validation in 140 LUAD patients revealed that high SUMO2-K11la expression correlated with reduced ferroptosis activity (4-HNE staining) and poorer prognosis (Fig. 3i-l), suggesting its utility as a prognostic biomarker.
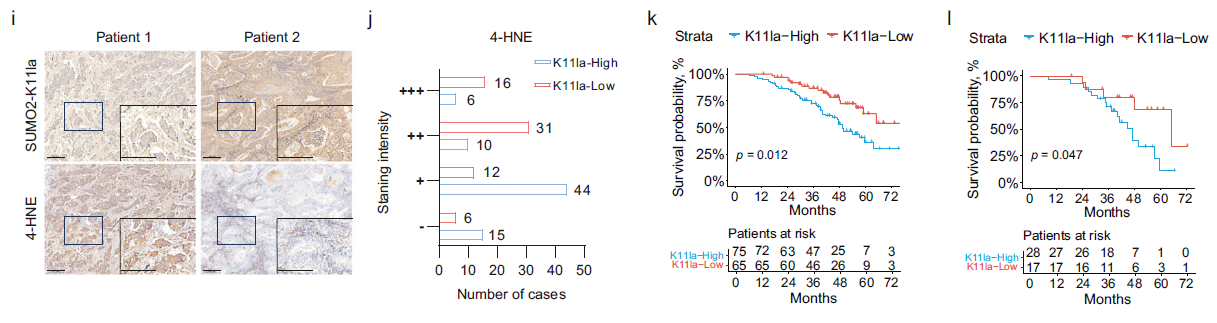
Step 3: Mechanistic Elucidation: SUMO2-K11la Promotes ACSL4 Ubiquitination-Mediated Degradation by Inhibiting ACSL4 Sumoylation
Central Question: How does SUMO2-K11la regulate ferroptosis? SUMO2 is a core protein in small ubiquitin-like modifier (SUMO) conjugation (sumoylation), while ACSL4 is a key driver enzyme of ferroptosis (promoting polyunsaturated fatty acid synthesis and accelerating lipid peroxidation);
Experimental Validation:
a. Sumoylation proteomic analysis identified lysine 500 of ACSL4 as a critical SUMO2 modification site, with ferroptosis induction leading to significant reduction in sumoylation at this site (Fig. 4a-b);
b. Co-immunoprecipitation (Co-IP) and proximity ligation assays (PLA) confirmed that SUMO2-K11la sterically hinders the interaction between SUMO2 and ACSL4, thereby reducing ACSL4 sumoylation (Fig. 4c-e);
c. Subsequent experiments demonstrated that loss of ACSL4 sumoylation promotes its ubiquitin-dependent proteasomal degradation (rescuable by MG132), ultimately reducing lipid peroxidation and suppressing ferroptosis (Fig. 4g-i).
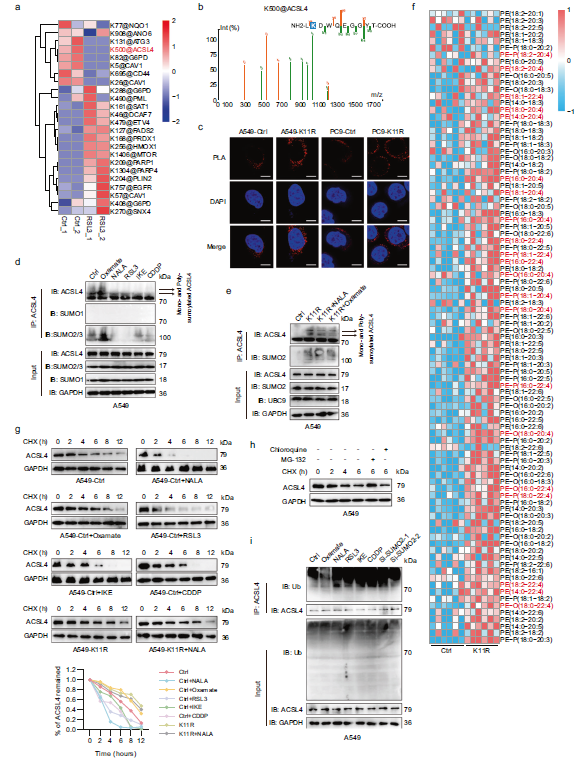
Step 4: Targeted Drug Development + Clinical Model Validation—From Bench to Bedside
Drug Design: Based on the SUMO2-K11 site sequence, a cell-penetrating peptide K11-Pep (containing the YGRKKRRQRRR penetration domain) was designed to competitively inhibit SUMO2-K11la;
Model Validation:
a. Cellular level: K11-Pep significantly enhanced LUAD cell sensitivity to ferroptosis inducers and CDDP (Fig. 7d-e);
b. PDO Model (Critical Support from Absin Matrigel): Patient-derived organoids were established from surgically resected tumor tissues using Absin Matrigel. H&E staining confirmed morphological consistency with primary tissues (Fig. 7f). Drug sensitivity assays demonstrated that K11-Pep increased PDO sensitivity to RSL3 and CDDP by 2-3 fold (Fig. 7g);
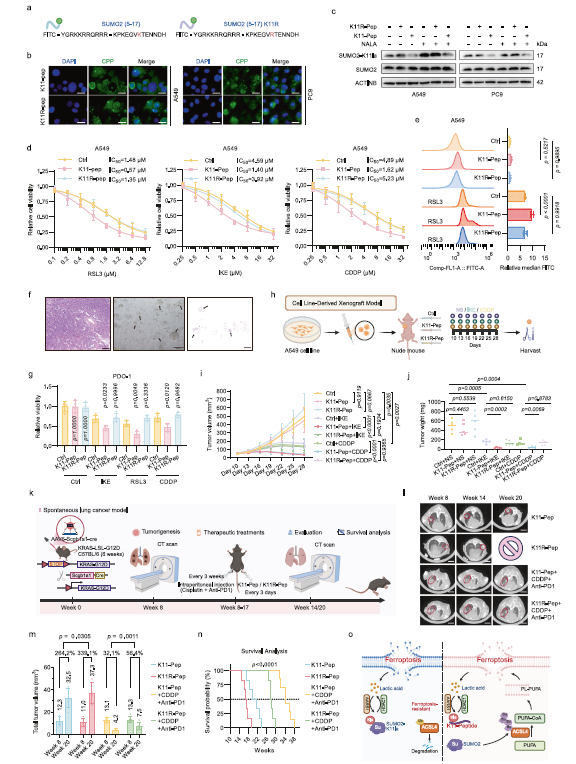
c. Animal models: In xenograft models, K11-Pep combined with IKE/CDDP significantly inhibited tumor growth (without apparent hepatorenal toxicity). In the spontaneous lung cancer model (KrasG12D mutation), K11-Pep combined with CDDP and anti-PD-1 therapy reduced tumor burden and extended survival (Fig. 7i-n).
III. Absin Product Highlight: Matrigel (abs9493)—The "Gold Standard" for PDO Model Construction
In this study, the PDO model served as the critical bridge connecting basic research with clinical application. Absin's Matrigel product (Catalog No.: abs9493) provided essential support for successful PDO establishment:
1. Product Application: In Vitro Culture and Drug Sensitivity Testing of LUAD PDOs
Experimental Procedure: Fresh LUAD tissues were dissociated into single cells, resuspended in Absin Matrigel (1:25 dilution), plated, and overlaid with organoid culture medium. Following Matrigel solidification, drug treatments (K11-Pep + ferroptosis inducers/CDDP) were administered, and viability was assessed using CellTiter-Lumi (Fig. 7f-g);
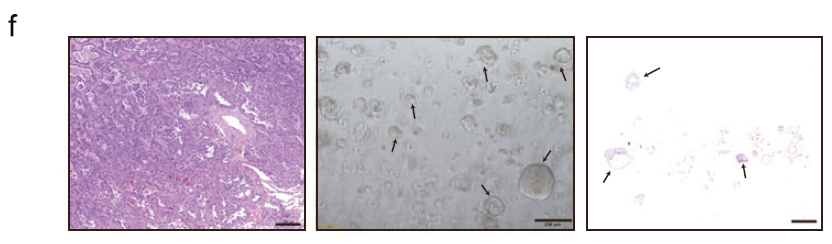

Critical Role: Matrigel mimics the extracellular matrix (ECM) architecture of the in vivo tumor microenvironment, providing structural support for three-dimensional growth, polarity maintenance, and functional differentiation of PDOs. This ensures that PDOs faithfully recapitulate the molecular characteristics and drug sensitivities of the primary tumors. In this study, PDOs cultured with Absin Matrigel not only exhibited morphological consistency with primary tissues (verified by H&E staining) but also accurately reflected patient tumor responses to K11-Pep, providing a reliable in vitro model for subsequent clinical translation.
2. Product Advantages: High Purity, High Reproducibility—Meeting Scientific and Clinical Translation Demands
High Activity: Protein concentration > 10 mg/mL with stable gelation strength, supporting long-term culture of diverse tumor organoids (lung, colorectal, breast, etc.);
Low Endotoxin: Endotoxin levels < 0.1 EU/mL, preventing cytotoxic effects;
High Consistency: Each batch is validated through cell growth promotion assays, ensuring experimental reproducibility (PDO culture success rate > 85% in this study).
IV. Research Summary: New Breakthroughs Bringing New Hope for LUAD Treatment
1. Mechanistic Breakthrough: First identification of SUMO2-K11la as a critical regulatory factor in LUAD ferroptosis resistance, revealing a novel pathway: "ferroptosis → glycolysis → SUMO2-K11la → ACSL4 degradation → ferroptosis resistance";
2. Target Breakthrough: SUMO2-K11la serves as a prognostic biomarker for LUAD (high expression correlates with shorter overall survival), and its regulated ACSL4-K500 sumoylation represents a critical node controlling ferroptosis sensitivity;
3. Therapeutic Breakthrough: The developed cell-penetrating peptide K11-Pep specifically targets SUMO2-K11la, demonstrating significant anti-tumor efficacy in cellular, PDO, and animal models. Combination with chemotherapy/immunotherapy further enhances therapeutic efficacy, laying the foundation for clinical translation;
4. Tool Breakthrough: Absin Matrigel products provided reliable support for PDO model construction, serving as a critical tool bridging basic research and clinical application.
This article is based on the original publication in Cell Discovery (DOI: 10.1038/s41421-025-00829-6). All images and data referenced herein are the intellectual property of the original journal and research team. Should any infringement occur, please contact us promptly for removal, and we will cooperate fully to resolve the matter.
Products Used in This Study
| Cat. No. | Name | Size |
| abs9493 | HC OrganoGel Phenol red free | 1.5mL×4/1.5mL×8 |
Contact Absin
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
| Absin Bioscience Inc. worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
