- Cart 0
- English
[IF 15.2] Absin Products Facilitate PD Mechanism Research: Novel Discovery of Mitochondrial Dysfunction Induced by Disrupted AMPK-SENP1-Sirt3 Signaling
February 04, 2026
Clicks:78
Parkinson's disease (PD) is a progressive neurodegenerative disorder with complex and incompletely elucidated pathogenesis. A recent study published in Translational Neurodegeneration provides critical new insights into PD pathogenesis by revealing how disrupted AMPK-SENP1-Sirt3 signaling impairs mitochondrial complex I (CI) function in the MPTP-induced PD model.
Title: Deficient AMPK-SENP1-Sirt3 signaling impairs mitochondrial complex I function in Parkinson's disease model
Journal: Transl Neurodegener (IF=15.2)
DOI: https://doi.org/10.1186/s40035-025-00489-2
Absin Products Used: Mouse IL-6 ELISA Kit (abs520004), Mouse TNF-α ELISA Kit (abs520010)

I. Research Rationale: Layered Investigation Focusing on Signaling Pathway-Mitochondrial Function Interactions
(1) Defining the Core Scientific Question
The neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is commonly employed to induce PD models, disrupting mitochondrial CI function and causing nigrostriatal dopaminergic neuronal loss. However, the specific molecular pathways through which MPTP damages mitochondrial CI function remain poorly characterized. Therefore, the research team aimed to identify the molecular mechanisms by which MPTP regulates CI function and determine the specific CI subunits affected by MPTP exposure.
(2) Designing In Vivo and In Vitro Validation Systems
- In Vivo Experiments: Wild-type Sirt3 and Sirt3 K223R desumoylation mutant male mice were utilized. PD models were established via intraperitoneal injection of MPTP or physiological saline, followed by assessment of motor performance, mitochondrial function, and protein acetylation levels.
- In Vitro Experiments: SH-SY5Y cell lines with or without Sirt3 desumoylation mutations were employed to simulate MPTP-induced environments for in-depth mechanistic investigation. This combined in vivo and in vitro experimental design enables comprehensive and accurate validation of the relevant signaling pathways.
(3) Focusing on Key Signaling Pathways and Molecular Modifications
The research team focused on the AMPK-SENP1-Sirt3 signaling cascade, with particular emphasis on how SUMOylation modifications affect Sirt3 deacetylase activity and subsequently influence mitochondrial CI subunit acetylation levels and CI function, progressively dissecting the molecular chain of MPTP-induced PD pathogenesis.
II. Research Findings: Disrupted AMPK-SENP1-Sirt3 Signaling as a Key PD Pathogenic Mechanism
(1) MPTP Disrupts the AMPK-SENP1-Sirt3 Axis, Triggering Mitochondrial Dysfunction
Both in vivo and in vitro experiments demonstrated that MPTP exposure inhibits AMPK activation and impedes SENP1 mitochondrial translocation. Deficiency of mitochondrial SENP1 leads to elevated Sirt3 SUMOylation levels, thereby suppressing its deacetylase activity. These sequential alterations result in significantly increased acetylation of CI subunits NDUFS3 and NDUFA5, ultimately causing reduced CI activity, mitochondrial dysfunction, and dopaminergic neuronal death.
(2) Sirt3 Desumoylation Mutation Alleviates MPTP-Induced Pathological Damage
Constitutive Sirt3 desumoylation mutations (K223R in mice, K288R in humans) attenuated MPTP-induced mitochondrial disturbances while reducing dopaminergic neuronal loss and ameliorating behavioral deficits. This finding provides a potential molecular target for PD therapeutics.
(3) Supporting Experimental Data
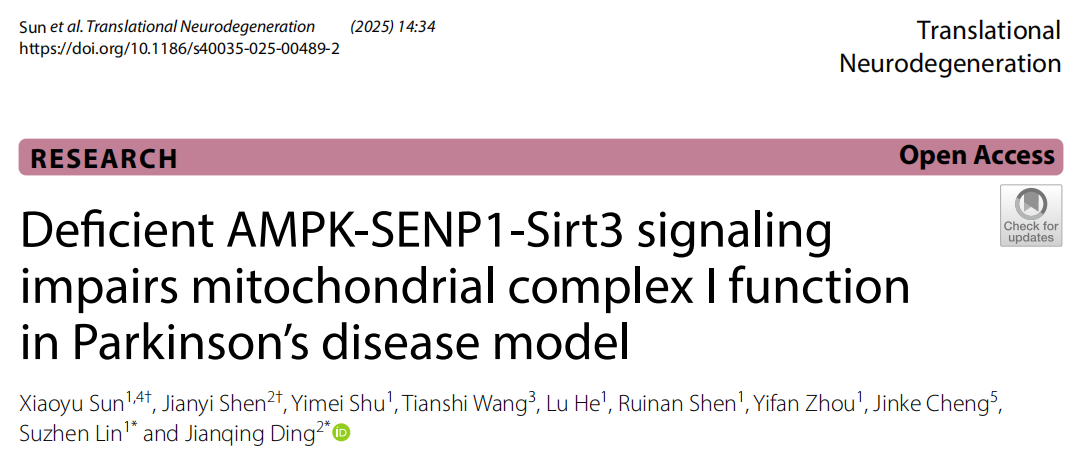
• Mitochondrial Protein Acetylation Levels: Following MPTP injection, acetylated protein levels in mouse ventral midbrain mitochondria were significantly elevated (P = 0.0016; Fig. 1b), indicating that MPTP alters mitochondrial protein acetylation status and potentially compromises function.
• Sirt3 SUMOylation Levels: Sirt3 SUMOylation levels in ventral midbrain mitochondria of MPTP-treated mice were significantly higher than in the saline group (P < 0.0001; Fig. 1c), demonstrating that MPTP-induced elevation in protein acetylation is closely associated with upregulated Sirt3 SUMOylation.
• CI Activity: Mitochondrial CI activity was significantly reduced in MPTP-treated mouse ventral midbrain and MPP+-treated SH-SY5Y cells (P = 0.0006, Fig. 1d; P = 0.0004, Fig. 1e, respectively), confirming the inhibitory effect of MPTP/MPP+ on CI activity.
• Cell Viability and Behavioral Assays: MPP+ treatment caused significant reduction in SH-SY5Y cell viability (P < 0.0001; Fig. 2c); meanwhile, Sirt3 K223R mutant mice exhibited superior motor performance (rotarod test, pole test, wire suspension test, and open field test; Fig. 5h-k) and reduced dopaminergic neuronal loss (Fig. 5a-g) following MPTP treatment.
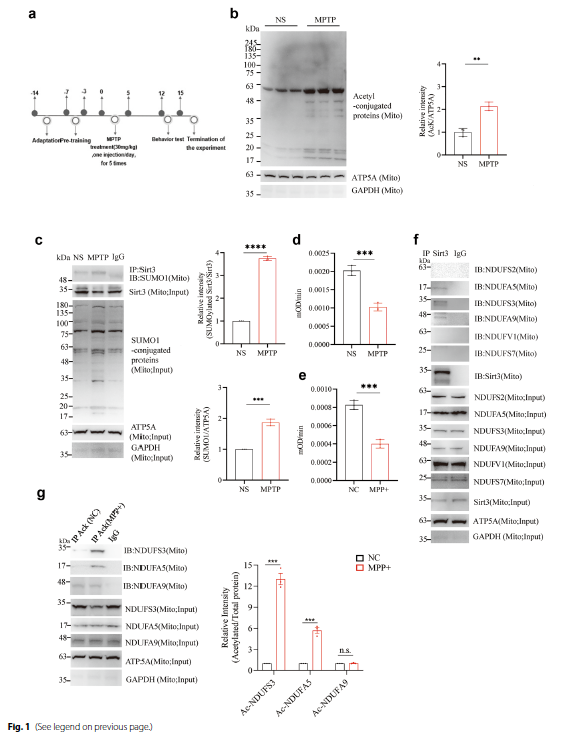
Fig.1 Detection of mitochondrial protein acetylation, Sirt3 SUMOylation, and CI activity
Fig.2 Cell viability and mouse behavioral experimental results
III. Absin Product Support: ELISA Kits for Precise Inflammatory Cytokine Detection
To evaluate neuroinflammatory responses in this study, the research team utilized Absin's IL-6 ELISA Kit (Catalog: abs520004) and TNF-α ELISA Kit (Catalog: abs520010) to detect levels of these inflammatory cytokines in cell culture supernatants.
(1) Product Application Scenarios
Researchers conducted experiments in BV2 microglial cells, treating cells with sub-threshold concentrations of MPP+ (0.01-0.02 mmol/L, insufficient to induce significant microglial activation or cytokine secretion) while silencing SENP1. Subsequently, IL-6 and TNF-α secretion was detected using Absin ELISA kits.
(2) Product Function and Experimental Value
- Precise Quantification: Absin ELISA kits exhibit high specificity and sensitivity, enabling accurate detection of low-concentration IL-6 and TNF-α in cell supernatants, providing reliable quantitative data for assessing microglial inflammatory responses.
- Pathway Function Validation: Experimental results demonstrated that SENP1 silencing significantly increased IL-6 and TNF-α secretion in BV2 cells even under sub-threshold MPP+ treatment (Fig. S2). This finding robustly establishes the critical role of the AMPK-SENP1-Sirt3 pathway in suppressing microglial inflammatory responses, further elucidating its function in PD pathogenesis.
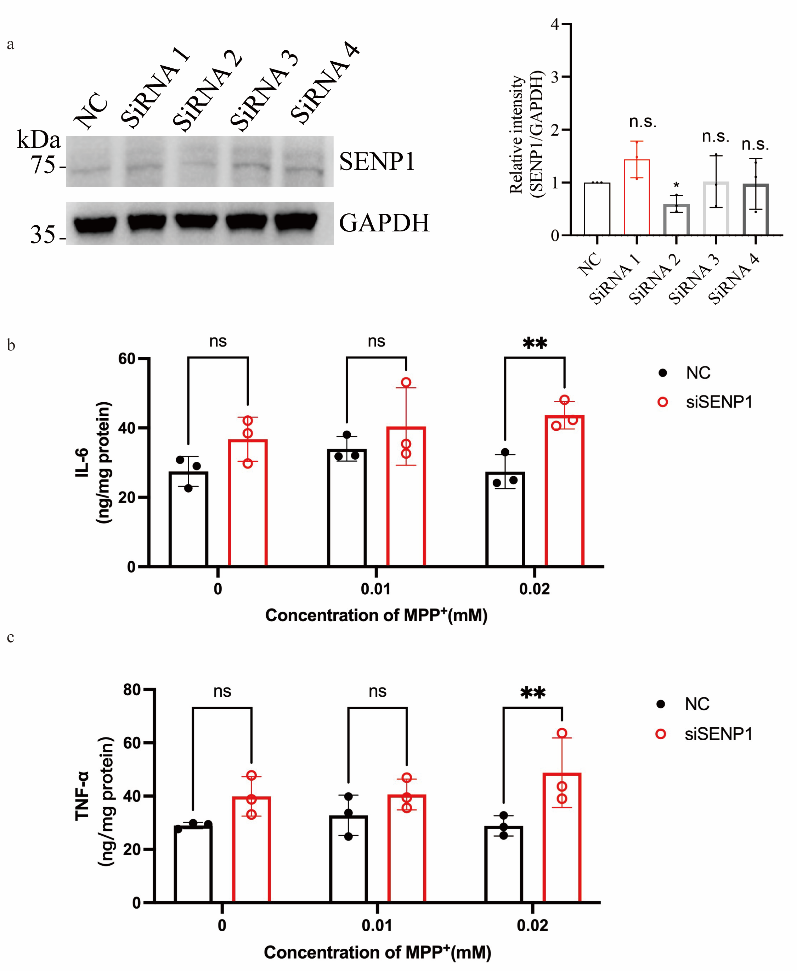
Fig. S2 Silencing SENP1 exacerbates the secretion of pro-inflammatory cytokines IL-6 and TNF-α in MPP+-treated microglia.
Absin remains committed to providing high-quality experimental reagents for researchers. The successful application of IL-6 and TNF-α ELISA kits in this Parkinson's disease mechanism study once again validates the reliability and utility of Absin products in life science research. Moving forward, Absin will continue supporting cutting-edge scientific exploration, providing superior products and services for disease mechanism research, drug development, and related fields.
This content is based on the original publication in Transl Neurodegener (DOI: 10.1186/s40035-025-00489-2). All images and data referenced herein are the intellectual property of the original journal and research team. Should any infringement occur, please contact us promptly for removal, and we will cooperate fully to address the matter.
Contact Absin
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
| Absin Bioscience Inc. worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
