- Cart 0
- English
Targeting Ceramide Transfer Protein Overcomes Therapeutic Resistance: HPA-12 Synergizes with FLT3 Inhibitors to Enhance Anti-Leukemic Efficacy in AML
February 03, 2026
Clicks:72
Acute myeloid leukemia (AML), a highly aggressive hematological malignancy, has long posed a clinical challenge due to chemoresistance and relapse in patients harboring FLT3 mutations. A recent landmark study published in Nature Communications offers a novel therapeutic solution to this dilemma—targeting the ceramide transfer protein (CERT) significantly enhances the cytotoxic efficacy of FLT3 inhibitors against AML cells. Notably, high-quality research reagents from Absin played a pivotal role in mechanistic validation within this study, providing robust support for the research team's breakthrough discoveries.
Title: Targeting ceramide transfer protein sensitizes AML to FLT3 inhibitors via a GRP78-ATF6-CHOP axis
Authors: Xiaofan Sun, Yue Li, Juan Du, et al.
Journal: Nature Communications (IF 15.7)
DOI: https://doi.org/10.1038/s41467-025-56520-7
Absin Products Used: Seven-Color Multiplex Immunofluorescence Staining Kit (Mouse/Rabbit Universal Secondary Antibody) (abs50015)
I. Research Rationale: Targeting Lipid Metabolism to Overcome Therapeutic Resistance
Dysregulated sphingolipid metabolism represents a critical mechanism underlying tumor cell survival and therapeutic resistance. Ceramide (Cer), a key tumor-suppressive lipid, exhibits defective generation and metabolism in cancer cells, enabling evasion of chemotherapeutic killing. The ceramide transfer protein (CERT) facilitates Cer transport from the endoplasmic reticulum (ER) to the Golgi apparatus, directly modulating the equilibrium between Cer and sphingomyelin (SM).
Prior investigations have demonstrated that CERT targeting enhances chemosensitivity in solid tumors, though its role in AML remained elusive. Given that FLT3-mutant AML cells exhibit suppressed Cer biosynthesis, the research team hypothesized that CERT inhibition would block Cer clearance, promote intracellular Cer accumulation, and consequently potentiate the antineoplastic effects of FLT3 inhibitors. To test this hypothesis, the team systematically investigated the synergistic interaction between the CERT inhibitor HPA-12 and the FLT3 inhibitor Crenolanib (Creno), spanning cellular assays, animal models, and clinical specimens.
II. Core Research Findings: Three Major Breakthroughs Providing Novel Therapeutic Strategies for AML
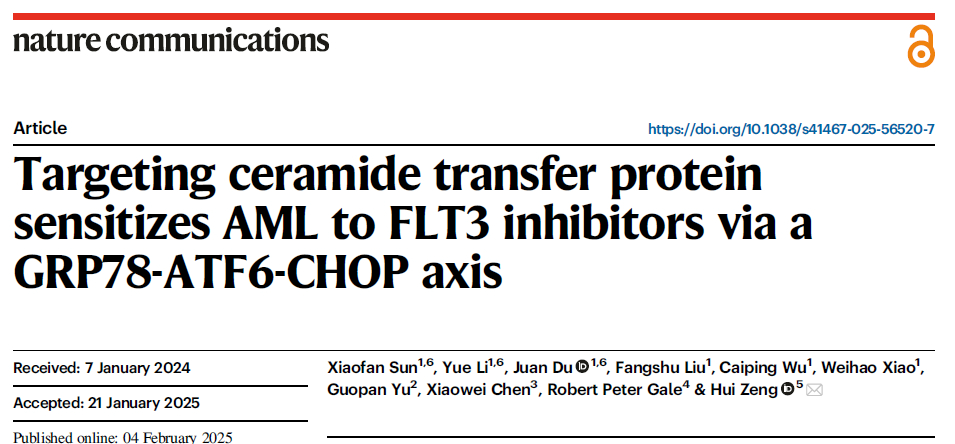
1. CERT as a Critical Therapeutic Target in FLT3-Mutant AML
Western blot analysis revealed significantly elevated CERT protein expression in FLT3-ITD mutant AML cell lines (MV4-11, Molm13) compared to wild-type AML cells and healthy donor bone marrow cells (Fig. 1a). Both pharmacological inhibition of CERT by HPA-12 and genetic knockdown via shRNA resulted in dose-dependent suppression of FLT3-mutant AML cell proliferation (Fig. 1c, d, f) and induction of apoptosis (Fig. 1g, h), with minimal cytotoxicity toward normal hematopoietic cells.
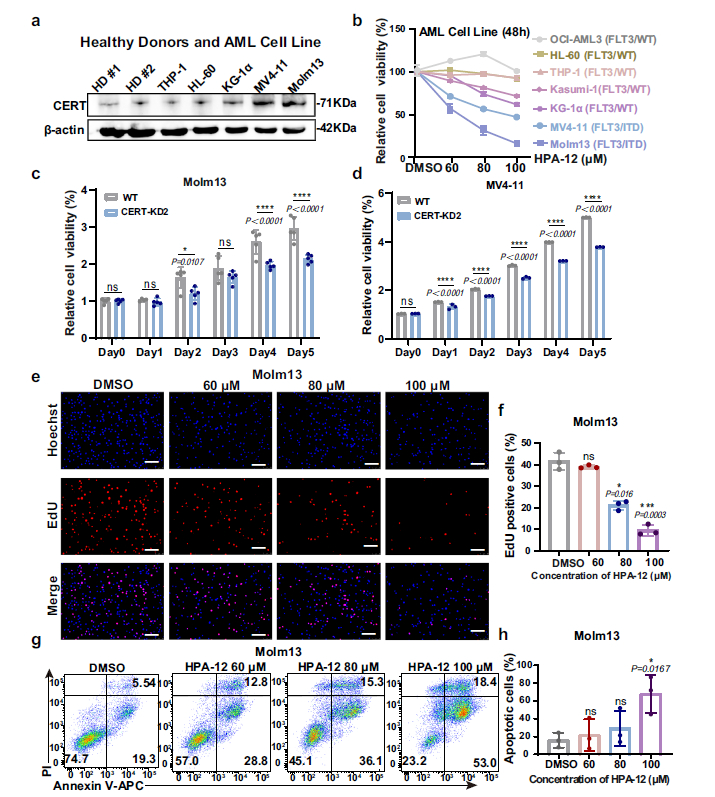
2. Synergistic Enhancement of HPA-12 and Creno Significantly Improves Therapeutic Efficacy
- In Vitro Experiments: Combined treatment with HPA-12 and Creno synergistically inhibited the viability of MV4-11 and Molm13 cells (combination index [CI] < 1, Fig. 2e, f), with significantly enhanced proliferation inhibition and apoptotic rates compared to monotherapy (Fig. 2h, j);
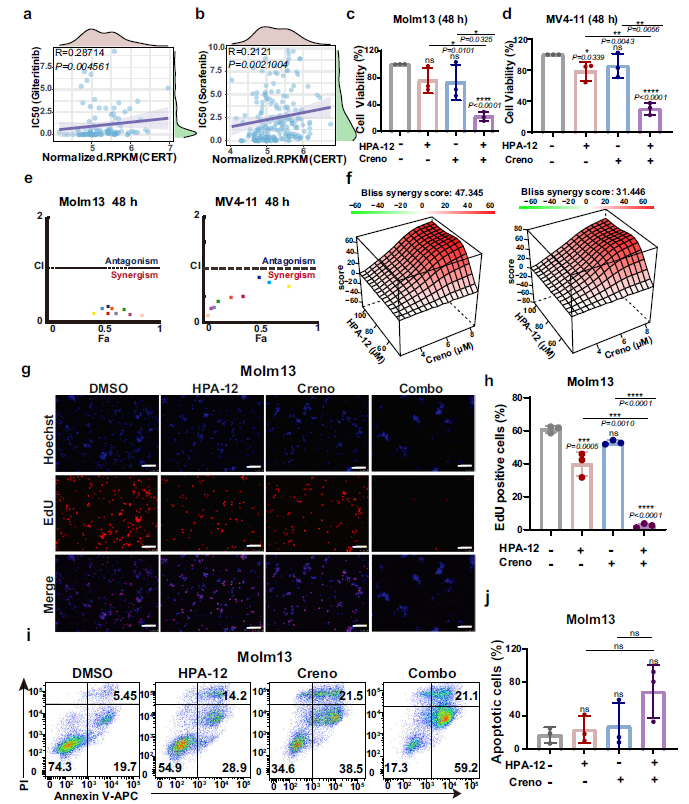
- In Vivo Experiments: In the MV4-11-luc+ xenograft model, combined therapy significantly reduced leukemia burden (Fig. 3b, f, g), ameliorated splenomegaly (Fig. 3d, e), and markedly prolonged survival (Fig. 3c);
- Clinical Sample Validation: Combined treatment effectively inhibited viability of FLT3-ITD+ and FLT3-TKD+ primary AML cells, activated apoptotic pathways, and exhibited minimal impact on healthy donor CD34+ hematopoietic stem cells (Fig. 9b, c).
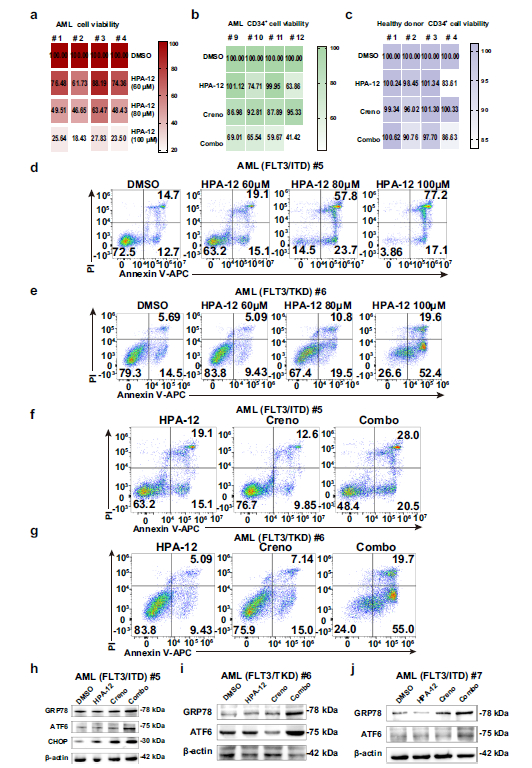
3. Molecular Mechanisms: GRP78-ATF6-CHOP Axis and Mitophagy Mediate Synergistic Effects
Combined therapy induces AML cell death through dual mechanisms:
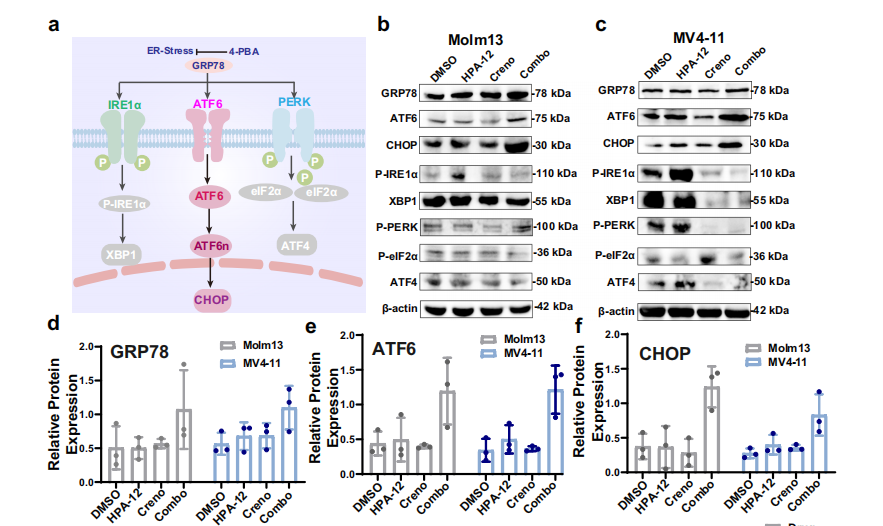
- Firstly, CERT inhibition blocks Cer transport while FLT3 inhibitors promote Cer generation, resulting in ER accumulation of Cer (Fig. 4e, g), triggering endoplasmic reticulum stress and specific activation of the GRP78-ATF6-CHOP pathway (Fig. 5b, d-f; Fig. 6);
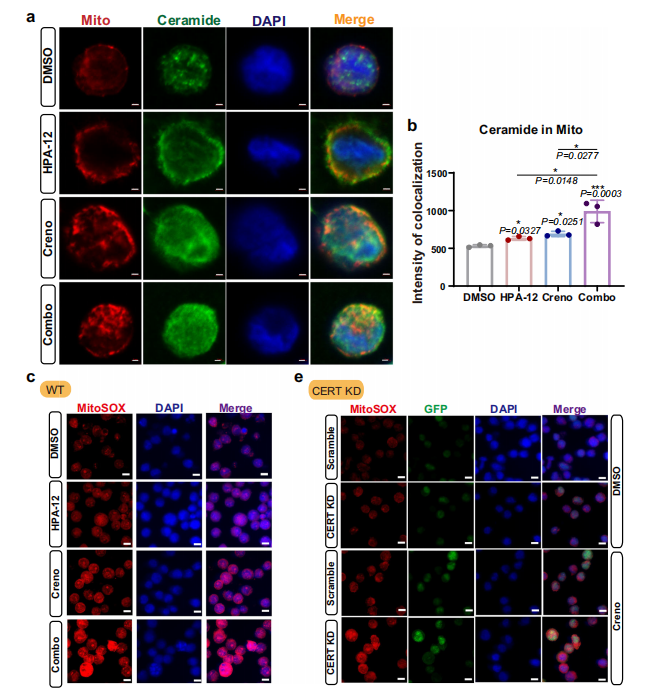
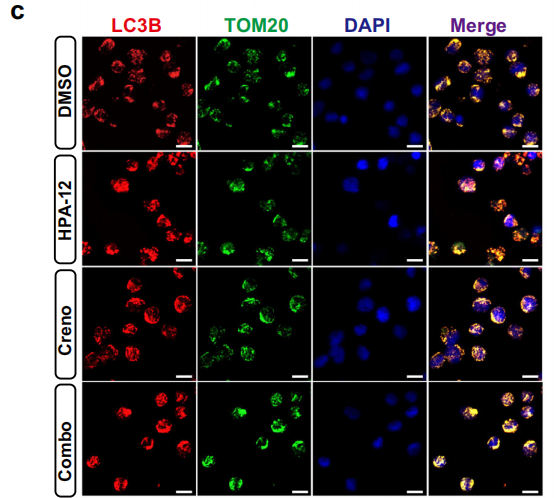
- Secondly, mitochondrial accumulation of Cer leads to decreased mitochondrial membrane potential and increased ROS generation (Fig. 7a-e), inducing mitophagy (Fig. 8a-c) and further enhancing cytotoxicity.
III. mIHC Detection Markers and Core Conclusions
1. Key mIHC Detection Markers
The study employed multiplex immunohistochemistry (mIHC) technology for precise localization of target molecules' subcellular distribution and expression levels. Core detection markers included:
Cellular Markers: Human CD45 (hCD45), human CD33 (hCD33), utilized for identification of leukemic cells in bone marrow and spleen (Fig. 3f, g; Fig. 3i);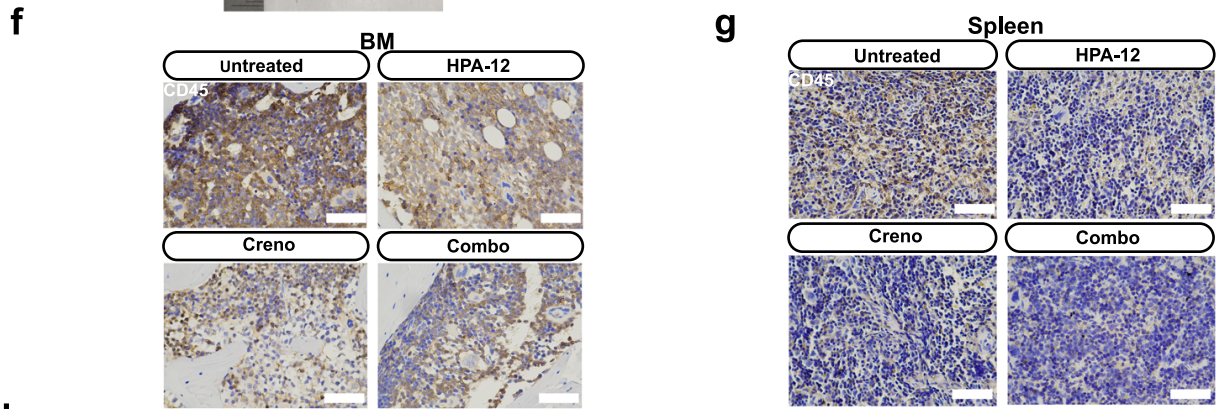
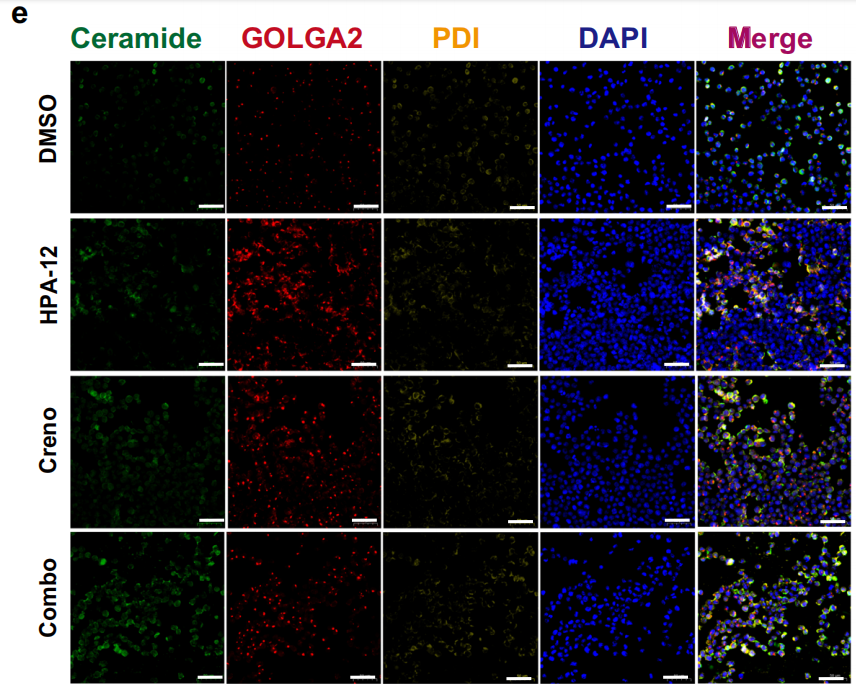
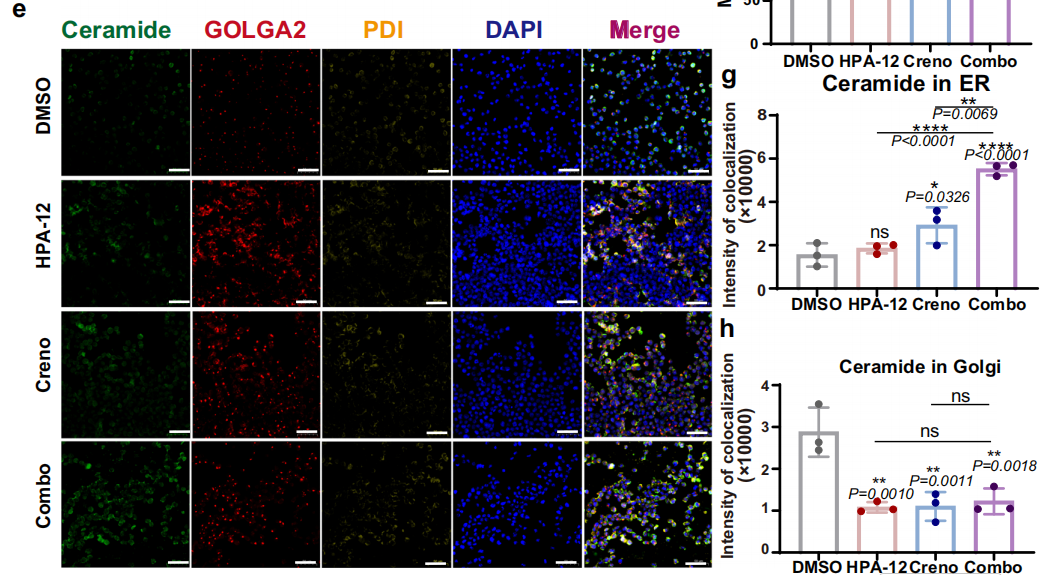
Subcellular Structure Markers: PDI (ER marker), GOLGA2/GM130 (Golgi apparatus marker), Tom20 (mitochondrial marker), utilized for definitive Cer subcellular localization (Fig. 4e; Fig. 7a; Fig. 8c);
 Signaling Pathway Molecules: GRP78, ATF6, CHOP, employed for detection of endoplasmic reticulum stress pathway activation status (Fig. 5b, c; Fig. 9h-j);
Signaling Pathway Molecules: GRP78, ATF6, CHOP, employed for detection of endoplasmic reticulum stress pathway activation status (Fig. 5b, c; Fig. 9h-j);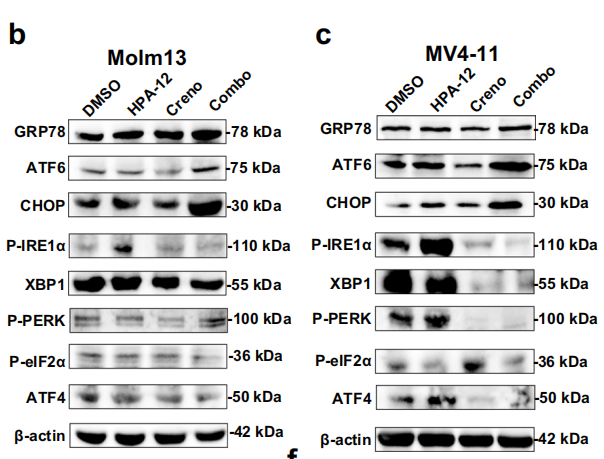
Autophagy-Related Markers: LC3B, utilized for validation of mitophagy occurrence (Fig. 8c).
2. Core mIHC Detection Conclusions
Leukemic Cell Localization: In xenograft mouse bone marrow and splenic tissues, mIHC staining demonstrated significantly reduced numbers of hCD45+, hCD33+ leukemic cells in the combination therapy group compared to monotherapy and control groups, directly confirming the in vivo anti-leukemic efficacy of combined treatment (Fig. 3f, g; Fig. 3i);Lipid Accumulation Localization: Through co-localization analysis of Cer with PDI and GOLGA2, the study confirmed that Cer predominantly accumulates in the ER following combined therapy, with reduced Cer content in the Golgi apparatus, validating the mechanism of impaired Cer transport following CERT inhibition (Fig. 4e, g, h);
Pathway Activation Validation: In FLT3-ITD+ and FLT3-TKD+ primary AML specimens, mIHC detected significantly upregulated protein expression of GRP78, ATF6, and CHOP, confirming that combined therapy activates the GRP78-ATF6-CHOP pathway (Fig. 9h-j);
Mitophagy Validation: Enhanced co-localization signals between LC3B and Tom20 indicated that combined therapy induces mitophagy in AML cells, participating in apoptotic regulation (Fig. 8c).
IV. Absin Product Support: Critical Experimental Tools Underpinning Mechanistic Validation
In this high-impact study, Absin's TSA 7-Color Fluorescence Staining Kit (abs50015) served as one of the core tools for mechanistic validation, directly supporting the acquisition of critical experimental evidence regarding "subcellular localization of Cer in the endoplasmic reticulum and Golgi apparatus."
Product Information and Applications
| Product Name | Catalog Number | Application |
|---|---|---|
| Seven-Color Multiplex Immunofluorescence Staining Kit (Mouse/Rabbit Universal Secondary Antibody) | abs50015 | Utilized for co-localization analysis of ceramide (Cer) with endoplasmic reticulum (PDI marker) and Golgi apparatus (GOLGA2/GM130 marker) in AML cells |
Core Role of the Product in the Study
To elucidate the subcellular distribution of Cer following combined therapy, the research team employed the Absin TSA 7-Color Fluorescence Staining Kit to perform triple immunofluorescence staining for Cer, PDI (ER marker), and GOLGA2 (Golgi apparatus marker) in MV4-11 cells. Confocal microscopy observations revealed:
- Significantly elevated co-localization intensity between Cer and the endoplasmic reticulum in the combination treatment group (Fig. 4e, g), confirming ER accumulation of Cer;
- Reduced co-localization intensity between Cer and the Golgi apparatus (Fig. 4h), validating the critical hypothesis of impaired Cer transport following CERT inhibition.
These experimental results directly supported the core mechanism of "Cer accumulation-triggered endoplasmic reticulum stress," laying the foundation for subsequent validation of GRP78-ATF6-CHOP pathway activation, and representing a critical experimental link connecting "phenotypic observation" with "mechanistic elucidation."
V. Summary and Perspectives
This study provides the first demonstration that targeting CERT enhances the therapeutic efficacy of FLT3 inhibitors in AML, offering a novel combination therapeutic strategy for FLT3-mutant AML patients. Through precise mIHC detection of multiple markers, the study clearly elucidated Cer subcellular localization, signaling pathway activation, and leukemic cell cytotoxicity, providing intuitive visual evidence for mechanistic validation. Absin's TSA 7-Color Fluorescence Staining Kit, leveraging its high sensitivity and multi-channel compatibility, successfully facilitated the research team's critical localization experiments, underscoring the essential supportive role of high-quality research tools in driving scientific breakthroughs.
This content is based on the original publication in Nature Communications (DOI: 10.1038/s41467-025-56520-7). All images and data referenced herein are the intellectual property of the original journal and research team. Should any infringement occur, please contact us promptly for removal, and we will cooperate fully to address the matter.
Contact Absin
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
| Absin Bioscience Inc. worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
