- Cart 0
- English
Mouse CD8⁺ T-Cell Isolation & Expansion Protocol: A Complete Bench-Guide
January 16, 2026
Clicks:119
Sorting Principle
Biotin-conjugated monoclonal antibodies are used to label non-target cells (non-CD8+ T cells); streptavidin-coated magnetic beads then deplete these labeled cells, yielding untouched mouse CD8+ T cells. A magnetic separator is required.
Reagents & Instruments
Mouse CD8+ T-cell Isolation Kit (negative selection) (abs50129)
Kit composition:
|
Component |
5×108 cells |
1×109 cells |
|
Biotin-Antibody Mix |
100 µL |
200 µL |
|
Streptavidin MagBeads |
1 mL |
2 mL |

Cell Magnetic Separator (multi-function) (abs90335)
Sorting Protocol
1. Prepare single-cell suspension: mash spleen through a 70 µm strainer into ice-cold PBS; collect cells in a 50 mL tube, centrifuge 500 × g, 5 min.
2. Discard supernatant, resuspend in 5 mL ACK red-cell lysis buffer, RT 5 min; add 20 mL PBS, centrifuge 500 × g, 5 min.
Note: adjust volume and time according to the lysis reagent used. A few residual erythrocytes do not affect purity or downstream assays.
3. Resuspend in PBS, filter through a 70 µm strainer, count cells, centrifuge 500 × g, 5 min.
Note: filtering is essential to remove tissue debris and clumps that would reduce purity.
4. Discard supernatant, resuspend in sorting buffer (PBS + 2 mM EDTA + 2 % FBS or 0.5 % BSA, sterile-filtered) at 1×108 cells/mL.
5. Add 100 µL cell suspension (1×107 cells) to the bottom of a 5 mL FACS tube; add 2 µL Biotin-Antibody Mix, mix gently, incubate 10 min on ice.
Note: scale antibody proportionally for larger cell numbers; pipette into tube bottom to avoid wall adhesion.
6. Add 20 µL pre-washed Streptavidin MagBeads (wash 2× with sorting buffer: vortex, add 1 mL buffer, 10 000 × g 1 min, discard supernatant, resuspend in original volume), mix, incubate 10 min on ice.
Note: for 5×107 cells use 10 µL antibody + 100 µL beads; for <1×107 cells keep final volume at 100 µL.
7. Add 2.5 mL sorting buffer, pipette up-down 5× (no vortex).
8. Place tube on magnet for 5 min.
9. Decant supernatant gently into a new tube (keep tube on magnet); this fraction contains untouched CD8+ T cells. Centrifuge 500 × g, 5 min, discard supernatant.
10. Wash once, resuspend in desired buffer or medium for downstream assays.
Sorting Performance
C57BL/6 splenocytes were stained with FITC anti-mouse CD8 (clone 53-6.7). Purity increased from 13.8 % pre-sort to 97.1 % post-sort.
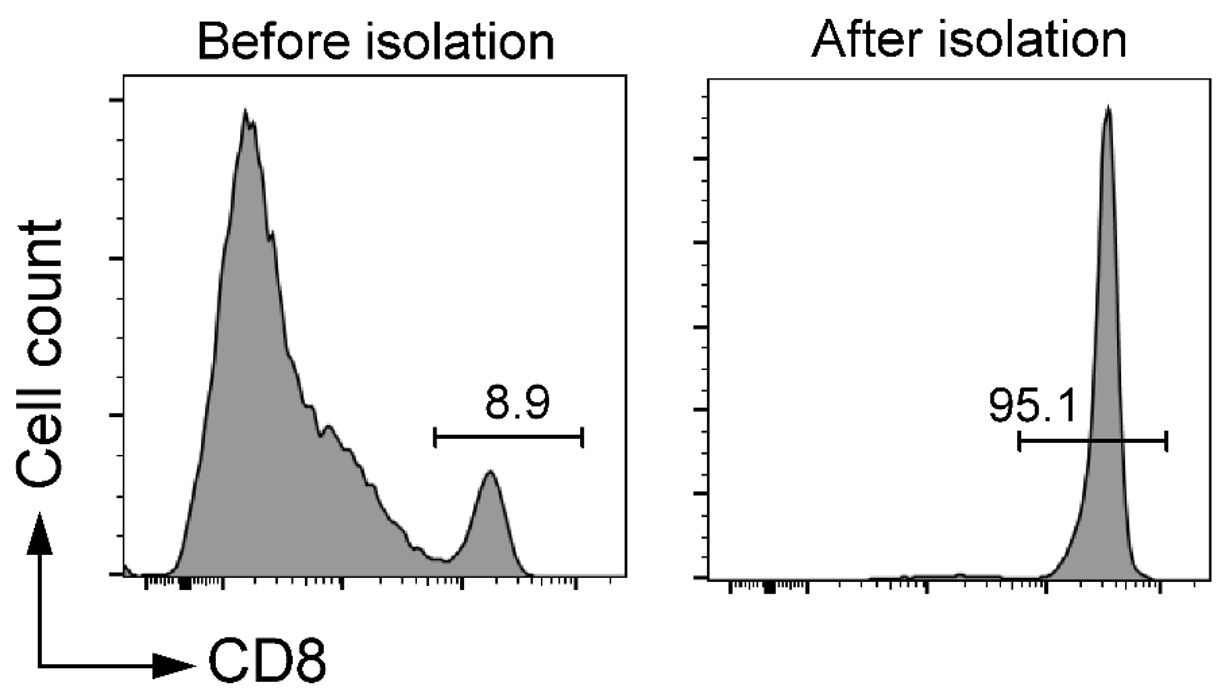
Independent replicate using clone 53-5.8: pre-sort 8.9 %, post-sort 95.1 % CD8+ cells.
Culture Principle
For short-term maintenance (3–5 d metabolic activity) without large-scale expansion useMouse Spleen CD8+ T-cell Maintenance Medium (abs90125).
For robust expansion combine:
Mouse Spleen CD8+ T-cell Maintenance Medium (abs90125) + Anti-Mouse CD3/CD28 Monoclonal Antibody Beads (Activation) (abs160030)
The 5 µm super-paramagnetic beads are covalently coupled with anti-CD3 and anti-CD28 antibodies, providing artificial APC-like signals without feeder cells; beads are removed magnetically after activation/expansion.
Culture Protocol
I. Reagent preparation
1. Wash buffer: PBS + 2 mM EDTA + 2 % FBS or 0.5 % BSA, sterile-filtered (0.22 µm).
2. Mouse Spleen CD8+ T-cell Maintenance Medium (abs90125)
3. Anti-Mouse CD3/CD28 Monoclonal Antibody Beads (Activation) (abs160030)
Note: vortex beads >30 s or rotate 5 min before use; avoid bubbles during pipetting. Remove beads with magnet before flow cytometry.
II. Procedure
1. Bead washing
(1) Resuspend stock beads thoroughly.
(2) Transfer desired volume to a tube, add equal volume wash buffer (or medium); vortex 5 s.
(3) Place on magnet 3 min, discard supernatant.
(4) Repeat wash once with medium.
(5) Resuspend in original volume of medium (e.g., 25 µL beads → wash → resuspend in 1 mL medium = 10× 96-well plate wells).
2. Activation (96-well plate format)
(1) Adjust T cells to 1×107 cells/mL; add 25 µL (2.5×105 cells) per well + 75 µL medium (total 100 µL).
(2) Add 100 µL washed bead suspension (bead:cell = 1:1); final volume 200 µL. Mix gently.
(3) Incubate 37 °C, 5 % CO2 for 24–48 h.
3. Expansion
(1) At 48 h check activation; if viability >90 %, pipette gently and split or perform half-medium change (remove 100 µL, add 100 µL fresh medium).
(2) Continue culture 37 °C, 5 % CO2; monitor daily.
(3) Mouse T cells typically show robust proliferation days 4–12; clusters indicate activation. Slow growth or shrinkage suggests exhaustion.
Note: do not disturb cells during first 48 h. Change medium or split when colour turns yellow or density >2.5×106 cells/mL; reseed at 0.5–1×106 cells/mL.
Product List
|
Cat. No. |
Product |
Size |
|
1 mL / 2 mL |
Contact Absin
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
| Absin Bioscience Inc. worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
