- Cart 0
- English
High-Glucose Fuel for Breast Cancer? Absin Decodes the RCC2-Lactylation Switch
January 06, 2026
Clicks:115
As the most prevalent malignancy in women, breast cancer incidence continues to rise. The causal links between dietary sugars, metabolic reprogramming and tumorigenesis remain an intense focus of research. Now, a cutting-edge study in Advanced Science unveils the first evidence that high-glucose-induced lactylation of RCC2 fuels breast-cancer proliferation, while Absin’s flagship reagents provide the enabling technology to decode the mechanism.
Title: High Sugar Induced RCC2 Lactylation Drives Breast Cancer Tumorigenicity Through Upregulating MAD2L1
Journal: Advanced Science (IF 14.1) | DOI: https://doi.org/10.1002/advs.202415530
Absin products used: IP/Co-IP Kit (abs955), Organotial Human Breast-Cancer Organoid Culture Kit (abs9446)

I. Conceptual framework: tracing the “high-glucose → lactate → proliferation” axis
Central hypothesis
High dietary glucose boosts tumor glycolysis, elevates extracellular lactate, and increases global protein lactylation. Whether lactylation links metabolic flux to MAD2L1 up-regulation and mitotic drive in breast cancer was unknown.
Logic pipeline
- Verify that high glucose raises lactate levels and MAD2L1 expression;
- Identify the lactylated scaffold protein coupling metabolism to MAD2L1 (RCC2);
- Map the lactylation site (K124) and the responsible acyl-transferase (KAT2A);
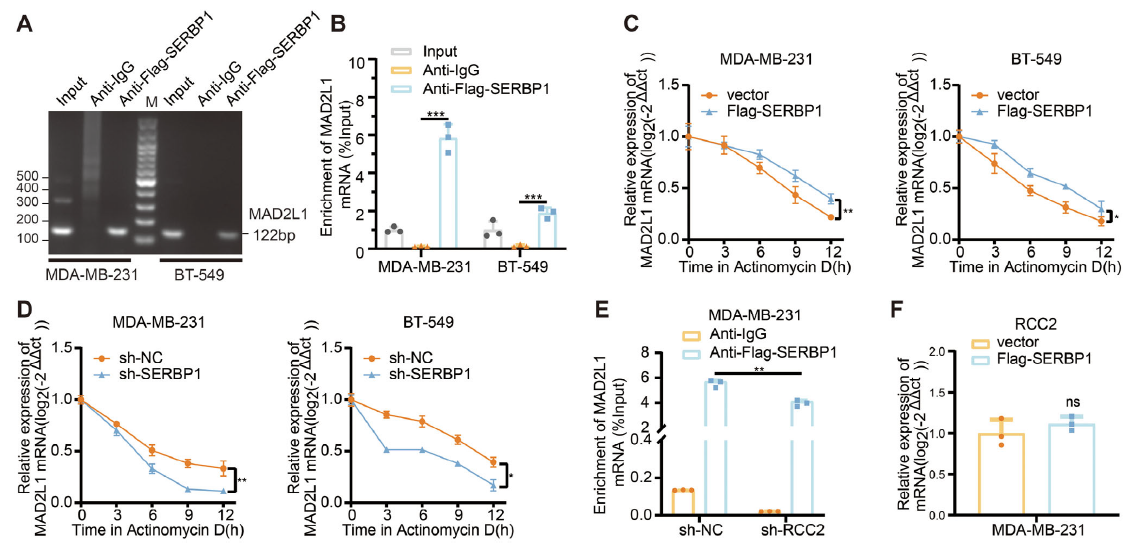
- Elucidate how RCC2-lactylation stabilizes MAD2L1 mRNA via SERBP1;
- Exploit the pathway therapeutically with a small-molecule RCC2-lactylation inhibitor.
II. Major breakthroughs: four milestones pointing to new therapeutics
1. High-glucose → lactate → MAD2L1 axis is essential for breast-cancer growth
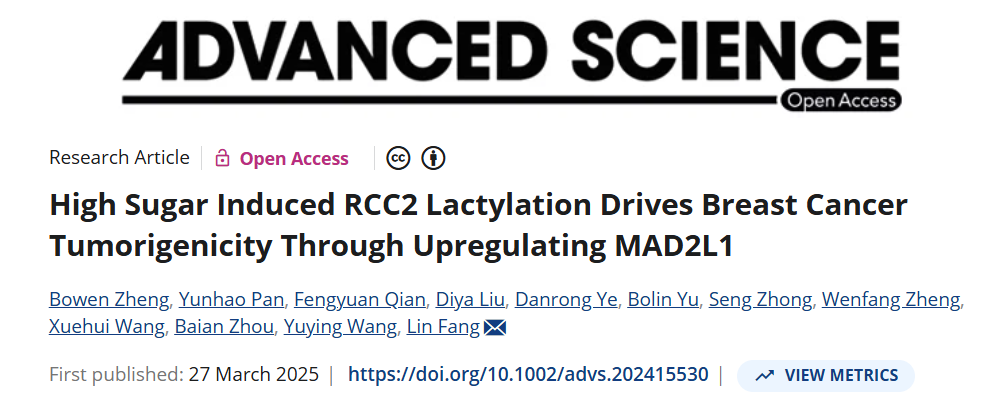
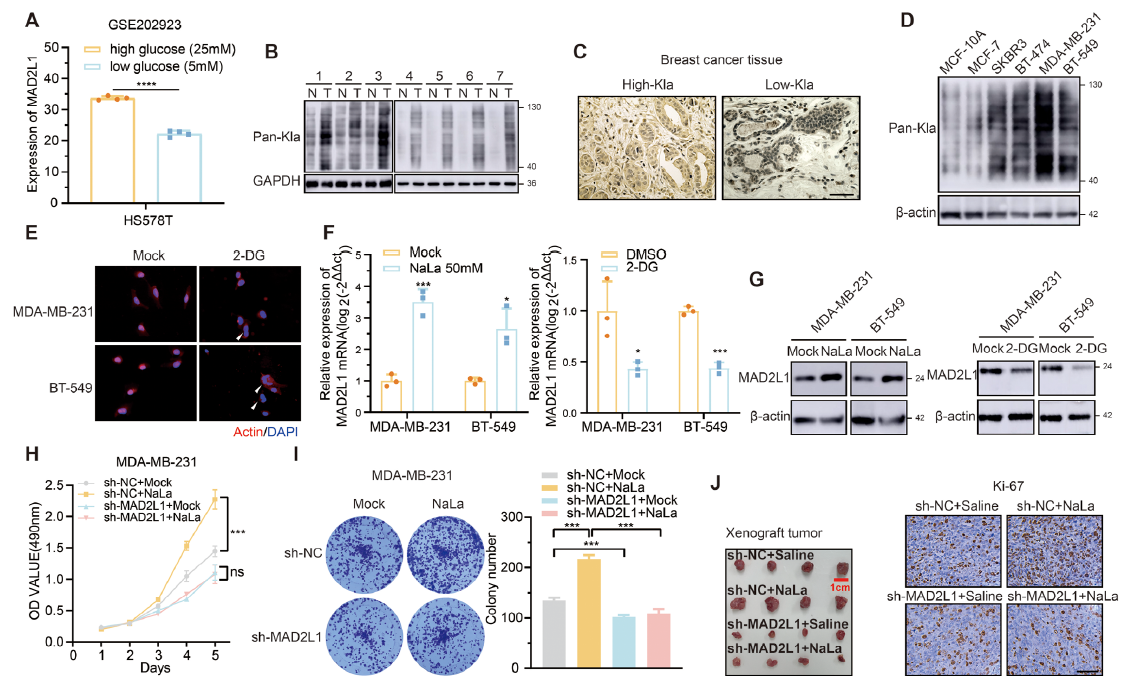
High-glucose culture increased global lactylation and MAD2L1 protein (Fig. 1B/C). Tumor size positively correlated with tissue lactylation (Table 1). Silencing MAD2L1 abolished lactate-induced proliferation (Fig. 1H–J), establishing MAD2L1 as the critical downstream effector.
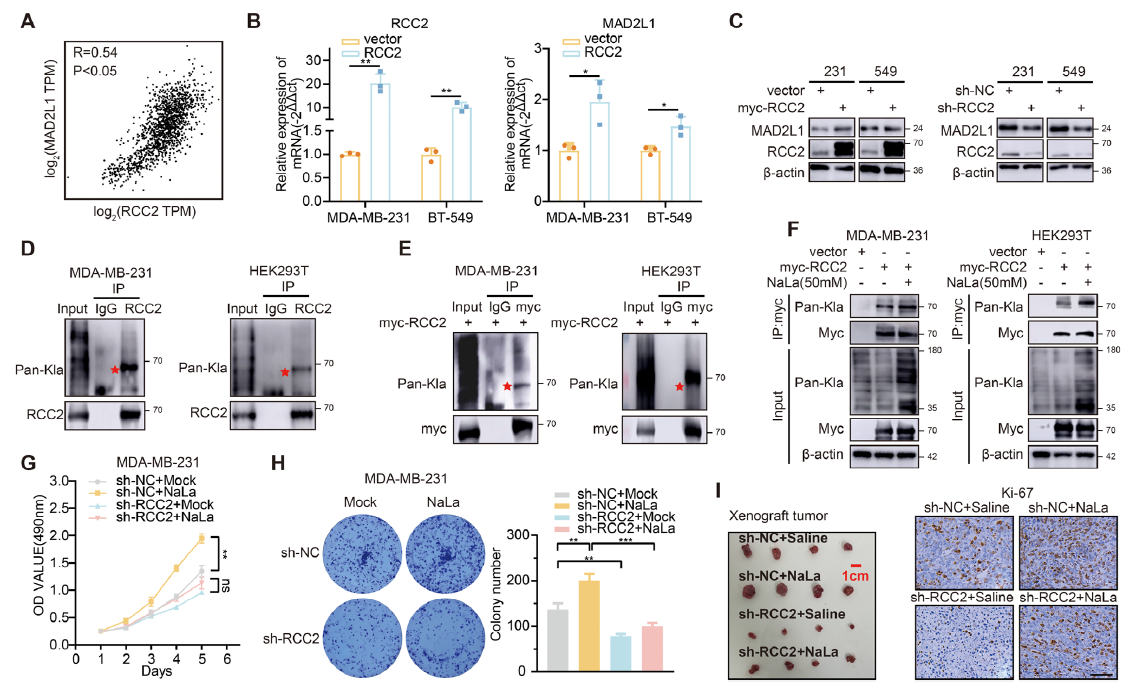
2. RCC2 is the principal lactylation substrate linking metabolism to MAD2L1
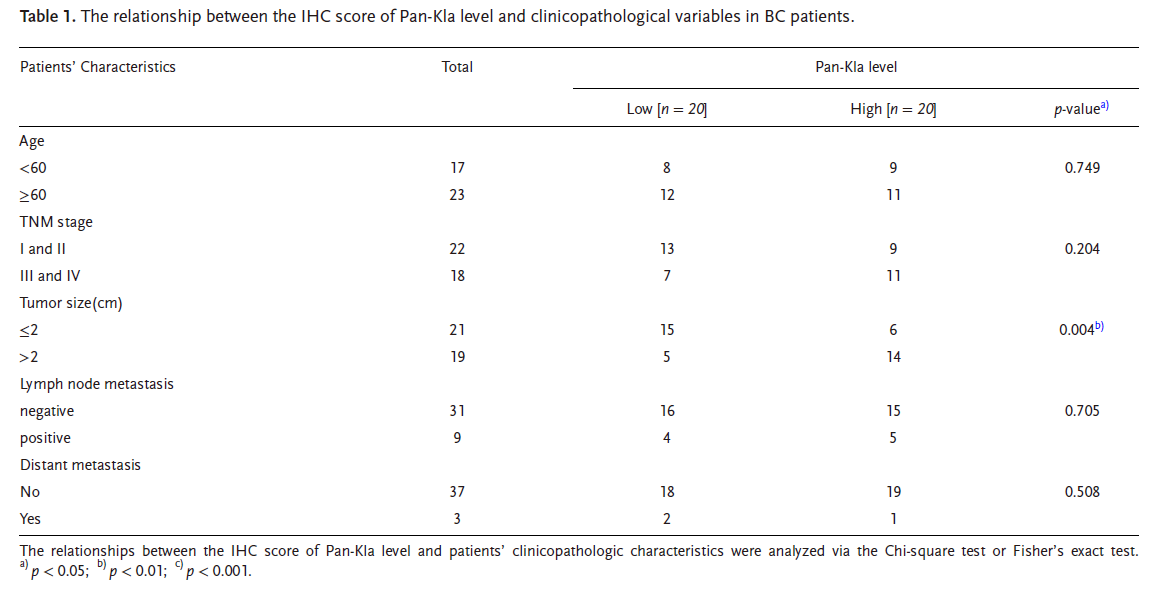
IP–MS identified RCC2 as a heavily lactylated protein (Fig. 2D-E). RCC2 and MAD2L1 expression were tightly correlated. RCC2 over-expression up-regulated MAD2L1, whereas RCC2 silencing reversed lactate-mediated induction of MAD2L1 and proliferation (Fig. 2B–C, G–I).
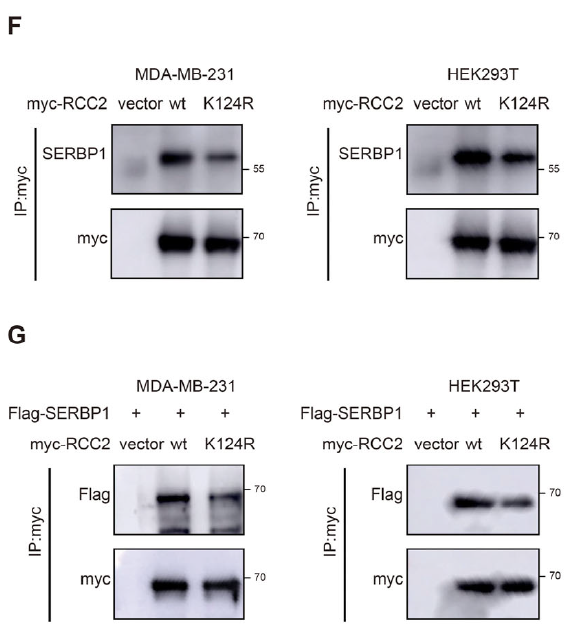
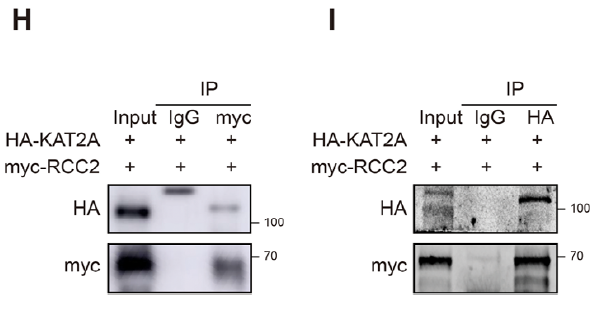
3. KAT2A lactylates RCC2 at K124, enhancing SERBP1 recruitment
Truncation mutagenesis and LC-MS pinpointed K124 as the conserved lactylation site (Fig. 3B–C, E). KAT2A was identified as the acyl-transferase catalyzing RCC2-K124-lactylation (Fig. 3F–G, K). Lactylation strengthened RCC2–SERBP1 association and promoted nuclear translocation of SERBP1 (Fig. 6F–J).

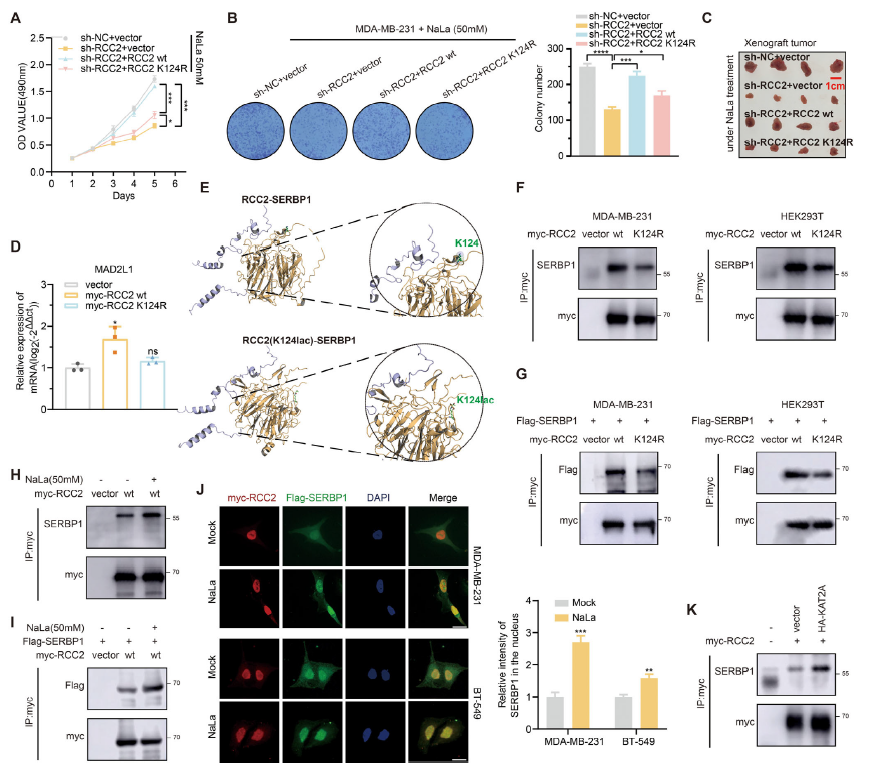
4. SERBP1 stabilizes MAD2L1 mRNA; SBDA blocks RCC2 lactylation and tumor growth
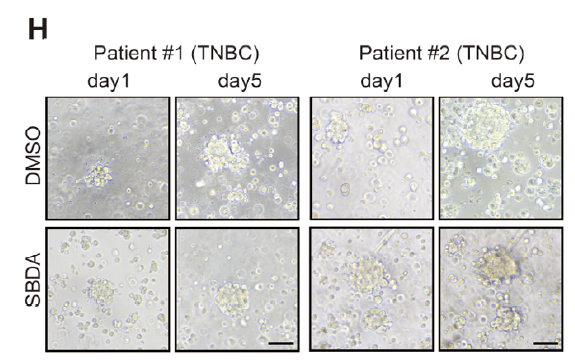
RIP confirmed SERBP1 binding to the 3′UTR of MAD2L1 mRNA, prolonging its half-life (Fig. 5A–D). RCC2-K124-lactylation was obligatory for this interaction (Fig. 5E). In-silico screening yielded SBDA, a small molecule occupying the RCC2-K124 pocket, which inhibited lactylation, reduced MAD2L1, and suppressed tumor growth in cells and patient-derived organoids (Fig. 7E–H).

III. Powered by absin: robust tools for decisive assays
Two absin products underpinned the mechanistic dissection:
Applications
- Detection of endogenous RCC2 lactylation (Fig. 2D): IP of RCC2 followed by Pan-Kla immunoblot;
- Validation of RCC2–SERBP1 interaction (Figs. 4G–H, 6F–G);
- Confirmation of KAT2A–RCC2 binding (Figs. 3H–I).
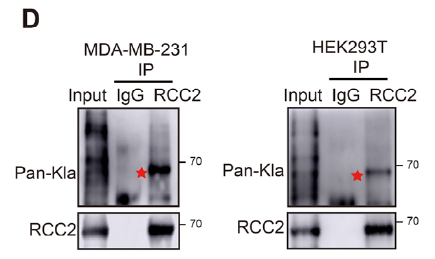
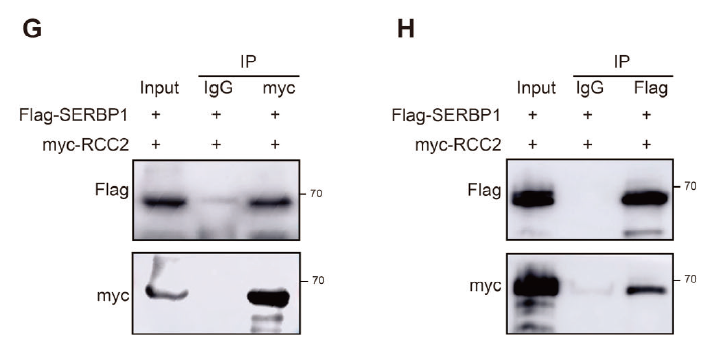
Key merits
Protein A/G magnetic beads deliver high binding capacity and low non-specific adsorption, ensuring clean inputs for downstream WB or MS.
Applications
Provided high-purity, genetically stable organoids for SBDA efficacy testing (Fig. 7H), bridging 2-D cultures and in-vivo models.
IV. Conclusions & perspectives
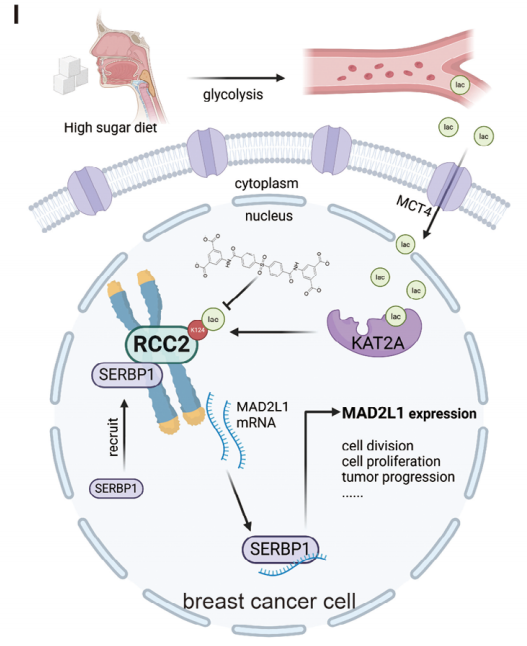
The study delineates a complete pathway: high glucose → lactate → KAT2A-mediated RCC2-K124 lactylation → SERBP1 nuclear import → MAD2L1 mRNA stabilization → breast-cancer proliferation (Fig. 7I). Small-molecule SBDA disrupts this oncogenic circuit, offering a tractable therapeutic avenue for sugar-associated breast cancers.
absin will continue to develop precise, user-friendly tools that empower researchers to unravel complex molecular networks and accelerate the translation of metabolic interventions into clinical benefit.
This summary is based on the open-access article published in Advanced Science (DOI: 10.1002/advs.202415530). All original figures and data are the intellectual property of the journal and the authors. If any infringement is suspected, please contact us for immediate removal; we will cooperate promptly and assume no legal liability.
|
Cat. # |
Product |
Size |
| Immunoprecipitation (IP/CoIP) kit | 50T | |
| Human Breast Cancer Organoid Culture Medium Kit | 1kit | |
| Protein A/G Magnetic IP/Co-IP Kit | 10T/50T | |
| abs50034 | ChIP Kit | 22T |
| abs50074 | DNA Pull Down Kit(Animal) | 6T |
| abs50072 | RNA Pull Down Kit | 6T |
Contact Absin
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
| Absin Bioscience Inc. worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
