- Cart 0
- English
Breaking the Protein-Delivery Bottleneck: Absin Recombinant Proteins Power Lipid Nanoparticles to Revolutionize Cancer Immunotherapy
December 29, 2025
Clicks:114
In the field of biopharmaceuticals, protein therapeutics—owing to their high potency and exquisite specificity—have become the cornerstone for treating major diseases such as cancer and autoimmune disorders. Nevertheless, intracellular delivery of protein drugs remains bottlenecked by low encapsulation efficiency, poor endosomal escape, and inadequate serum stability, severely constraining their clinical translation. A landmark study recently published in Advanced Science (DOI: 10.1002/advs.202500844) has now cracked this long-standing problem. The authors engineered a novel five-component lipid nanoparticle (LNP) platform that enables robust cytosolic delivery of diverse protein cargos, heralding a paradigm shift in cancer immunotherapy. Recombinant murine IL-10 protein from Absin served as a critical reagent throughout the study, providing definitive proof-of-concept for the LNP delivery system.
Title: Optimization of Lipid Nanoparticles with Robust Efficiency for the Delivery of Protein Therapeutics to Augment Cancer Immunotherapy
Journal: Advanced Science (IF 14.1)
DOI: https://doi.org/10.1002/advs.202500844
Absin product used: Mouse recombinant IL-10

I. Rationale: Precise Circumvention of Three Bottlenecks in Protein Delivery
Although conventional LNPs have proven formidable for nucleic-acid delivery (e.g., mRNA vaccines), their performance with proteins is dismal. The root causes lie in the charge heterogeneity, structural complexity, and serum lability of proteins, which collectively impede carrier association, endosomal escape, and bioactivity retention. To surmount these hurdles, the authors devised a synergistic “cationic + ionizable lipid” strategy:
- Formulation optimization: A five-component LNP library comprising cationic lipids (DOTMA/DOTAP), ionizable lipids (MC3/ALC-0315/SM-102), helper lipid (DOPE), cholesterol, and PEG-lipid was systematically screened to balance protein binding and endosomal disruption;
- Serum-stability engineering: PEGylation minimizes non-specific protein adsorption, while adsorbed serum albumin redirects LNPs toward albumin-receptor–mediated caveolar endocytosis, boosting in vivo delivery;
- Translatational focus: Therapeutically relevant proteins (saporin, IL-10, Cas9 RNP, IgG, SOD, CAT) were selected to benchmark LNP performance across cell lines, 3D tumor spheroids, and mouse tumor models.
II. Key Findings: End-to-End Breakthrough from Delivery to Therapy
1. High-efficiency intracellular delivery of diverse proteins with preserved bioactivity
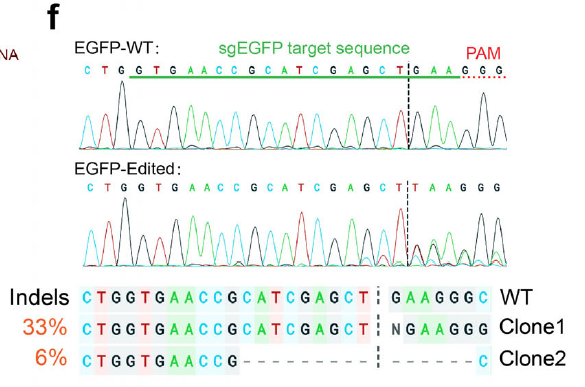
Optimized LNPs (DOTMA/MC3/DOPE 20:20:20 mol %) robustly delivered proteins spanning a wide MW and pI range, including IgG, SOD, CAT, saporin, and Cas9 RNP (Fig. 4). GFP-positive cells exceeded 80 %, outperforming the commercial reagent Pulsin, while delivered cargos retained native function: SOD quenched intracellular ROS, and Cas9 RNP achieved efficient gene editing (Fig. 4f).
2. Serum-enhanced delivery mechanism with superior tumor tropism
LNPs adsorb serum albumin, which redirects uptake via caveolin-1–dependent, albumin-receptor–mediated endocytosis (Fig. 3). This not only preserves serum stability but also exploits the overexpressed albumin receptors on tumors, leading to selective tumor accumulation. In 3D spheroids, Cy5.5-saporin-LNPs penetrated deeply, whereas free saporin was barely detectable (Fig. 5d).
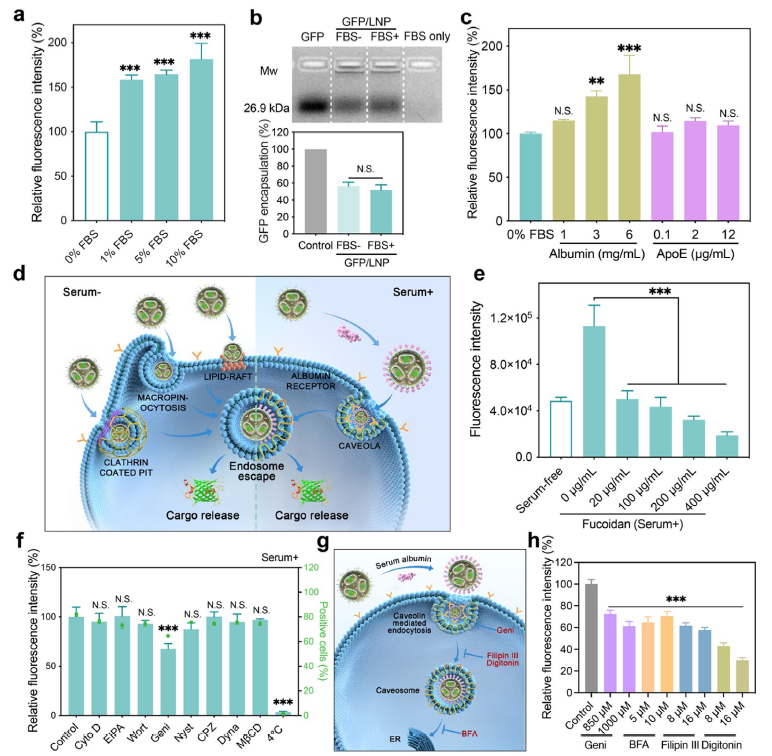
Serum-tolerance mechanism and intracellular trafficking of LNPs (original Figure 3)
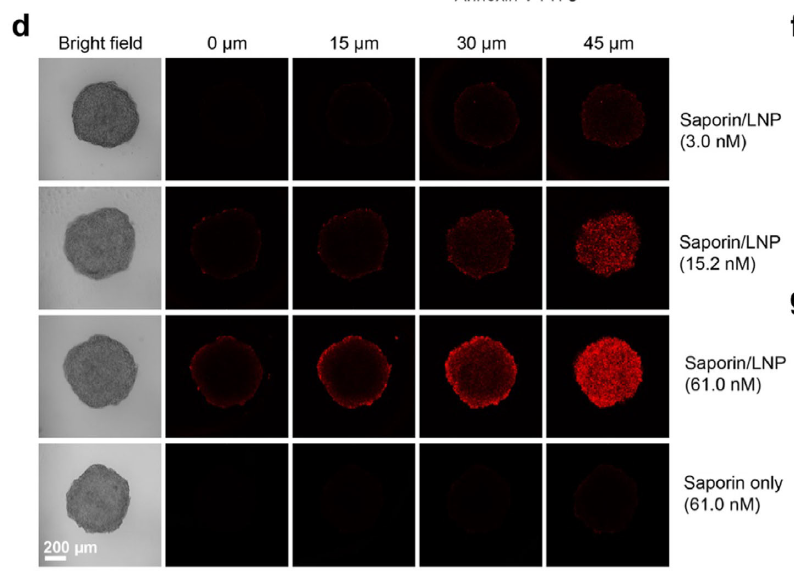
Penetration of saporin-LNPs in 3D tumor spheroids (original Figure 5d)
3. Therapeutic breakthrough: IL-10 delivery potentiates adoptive T-cell therapy
In a B16-OVA melanoma model, IL-10-LNPs extended the cytokine’s half-life and elevated intratumoral IL-10 levels. When combined with adoptively transferred OT-1 CD8+ T cells, tumor growth was markedly suppressed and mouse survival significantly prolonged (Fig. 6). Flow cytometry revealed increased infiltration of CD3+ and OT-1 CD8+ T cells, alongside elevated IFN-γ secretion, confirming robust immune activation.
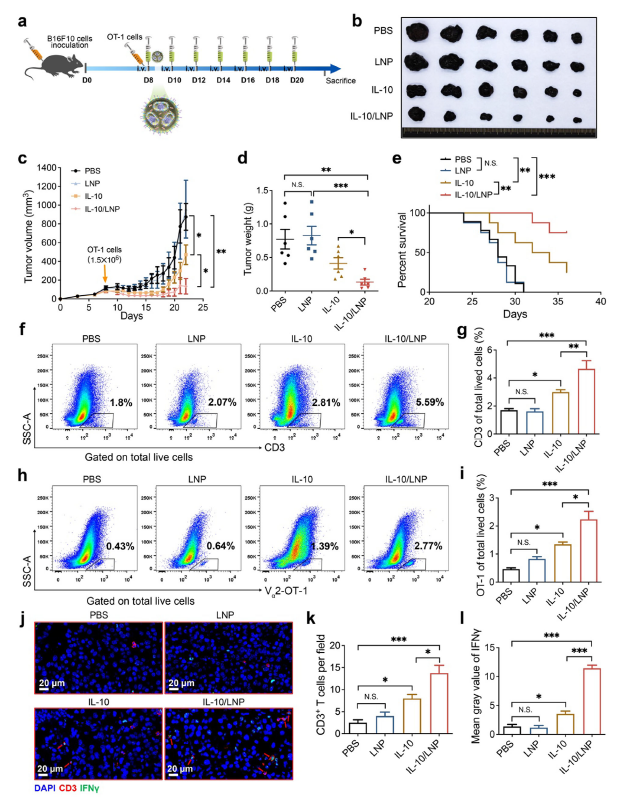
Therapeutic efficacy of IL-10-LNPs plus adoptive T-cell transfer against melanoma (original Figure 6)
III. Powered by Absin: Recombinant Murine IL-10 as the Functional Readout
Throughout this milestone study, Absin recombinant murine IL-10 underpinned both in-vitro and in-vivo validation of immunotherapeutic efficacy:
1. Applications
- In-vitro: quantification of LNP encapsulation efficiency and IL-10 bioactivity retention;
- In-vivo: formulation of IL-10-LNPs for intravenous administration and evaluation of therapeutic synergy with adoptive T-cell transfer in melanoma-bearing mice.
2. Key contributions
- As a well-defined immunomodulatory cytokine, Absin IL-10 provided an unambiguous functional readout—tumor IL-10 levels, T-cell proliferation, and IFN-γ secretion—directly corroborating LNP delivery efficiency and tumor targeting;
- High purity and potent bioactivity ensured reproducible, reliable data, solidifying the conclusion that “LNPs prolong cytokine half-life and amplify immunotherapeutic efficacy.”
| Product | Applications | Key role |
|---|---|---|
| Mouse recombinant IL-10 | In-vitro encapsulation assay; in-vivo immunotherapy | Functional readout; assay reliability & reproducibility |
IV. Conclusions & Perspectives
By rationally engineering a five-component LNP architecture, this study overcomes the long-standing technical barriers to intracellular protein delivery, expanding the utility of LNPs beyond nucleic acids and offering a versatile platform for cytokines, toxin proteins, and other “undeliverable” therapeutics. Absin is honored to have supplied the critical reagent that underpinned this breakthrough, and we remain committed to empowering scientists with premium products and services.
Content is based on the article published in Advanced Science (DOI: 10.1002/advs.202500844). All original figures and data are the intellectual property of the journal and the authors. Should any infringement arise, please contact us for immediate removal.
Contact Absin
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
| Absin Bioscience Inc. worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
