- Cart 0
- English
Breaking the TNBC Therapeutic Deadlock: Uncovering PCSK9’s Oncogenic Axis—Powered by Absin’s Next-Generation Antibodies
December 29, 2025
Clicks:155
Triple-negative breast cancer (TNBC), the most aggressive subtype of breast carcinoma, remains a clinical challenge due to the absence of well-defined molecular targets. A landmark study recently published in Advanced Science unveils, for the first time, that PCSK9 drives TNBC proliferation and metastasis by modulating plasma-membrane cholesterol to activate EGFR/HER3 signalling, thereby offering a novel therapeutic avenue. Absin antibodies provided critical analytical support throughout this breakthrough investigation.
Title: PCSK9 Promotes the Malignancy of Triple-negative Breast Cancer Cells by Reducing Cholesterol Levels at the Plasma Membrane to Activate EGFR and HER3
Journal: Advanced Science (IF 14.1)
DOI: https://doi.org/10.1002/advs.202408514
Absin reagent: Mouse anti-EGFR Monoclonal Antibody (abs149686)
![]()
I. Experimental rationale: pinpointing metastasis-driving genes in TNBC
- Establishment of a highly metastatic cell model: Parental MDA-MB-231-GFP TNBC cells were injected into the tail vein of nude mice; lung metastatic foci were isolated and expanded to generate the high-metastatic-potential sub-line 4-11.
- Transcriptomic screening: RNA-seq comparison between 4-11 and parental cells identified PCSK9 as significantly up-regulated in metastatic derivatives, a finding corroborated in TNBC cell lines and patient specimens.
- Functional validation of PCSK9: shRNA-mediated knock-down (shPCSK9) and over-expression (O-PCSK9) were combined with in-vitro (mammosphere, clonogenic, Transwell) and in-vivo (orthotopic implantation, experimental metastasis) assays to delineate PCSK9-dependent regulation of TNBC proliferation and metastasis.
- Mechanistic dissection: A step-wise investigation integrating cholesterol metabolism, lipid-raft integrity and signal-transduction revealed the sequential axis PCSK9→LDLR degradation→plasma-membrane cholesterol reduction→EGFR/HER3 activation→Src/ERK/c-Jun signalling.
- Clinical relevance: Public database mining and IHC staining demonstrated that high PCSK9 expression predicts poor prognosis in TNBC patients.
II. Key findings
1. PCSK9 is a critical driver of TNBC metastasis
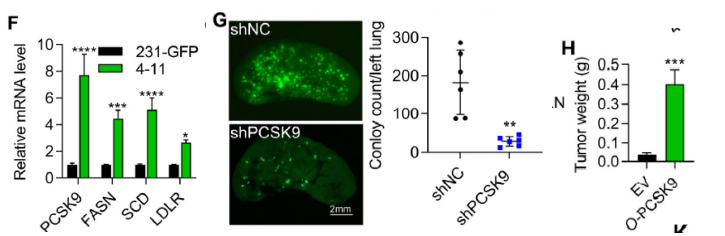
PCSK9 mRNA and protein levels were elevated 7.7- and 7.8-fold, respectively, in 4-11 cells. PCSK9 knock-down reduced lung metastatic burden by >90 %, whereas over-expression increased primary tumour mass 10-fold (Fig. 2F, 3G, 4H).
2. A novel pro-tumourigenic mechanism elucidated
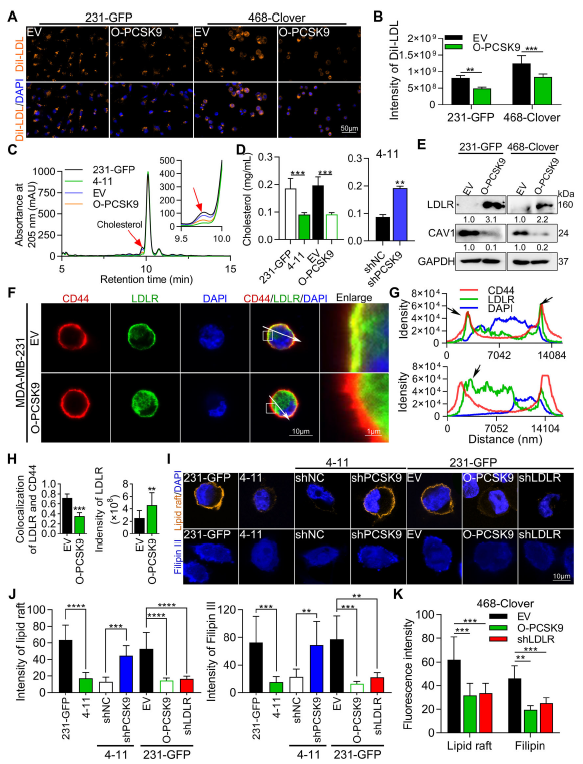
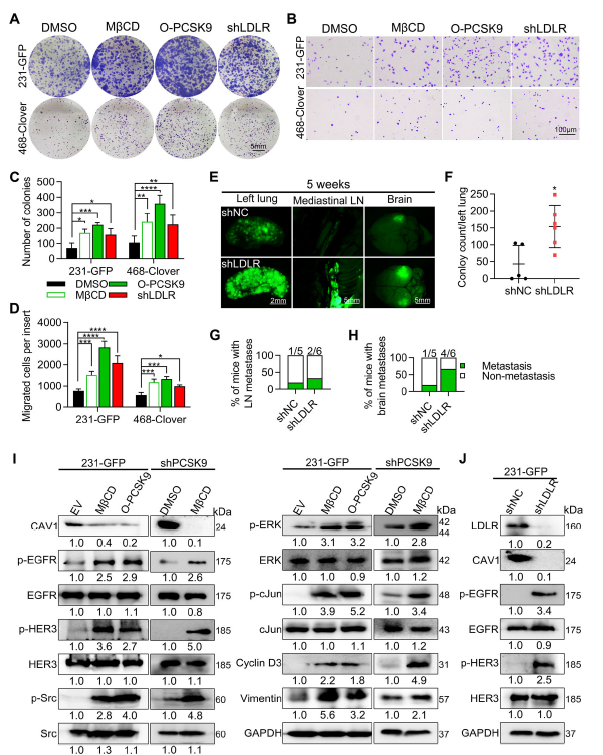
PCSK9 binds LDLR, promoting its lysosomal degradation, thereby decreasing membrane cholesterol and lipid-raft abundance. Raft disintegration relieves EGFR/HER3 inhibition, enabling homo- and hetero-dimerisation that triggers downstream signalling and up-regulates cyclin D3 and vimentin (Fig. 7, 8, 9G).
3. Translational relevance
PCSK9 expression is significantly higher in TNBC than in non-TNBC tumours; high expression correlates with shorter overall and distant-metastasis-free survival, positioning PCSK9 as a promising therapeutic target (Fig. 2J, 9A-B).
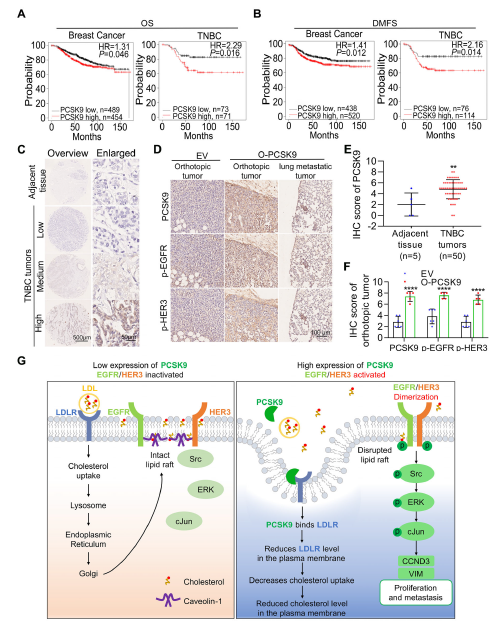
III. Absin products empowering pivotal experiments
The high specificity and robust stability of Absin’s EGFR antibody (cat. no. abs149686) enabled reliable Western-blot detection of total EGFR, providing a critical read-out for pathway interrogation.
1. Application
Quantification of total EGFR protein to determine whether PCSK9 modulates EGFR/HER3 signalling via expression changes (Fig. 5A, 8I-J).
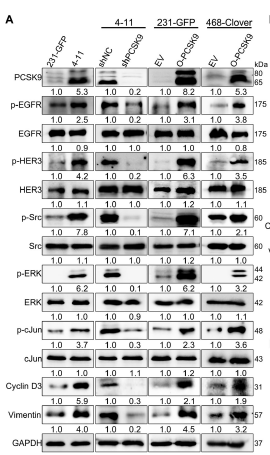
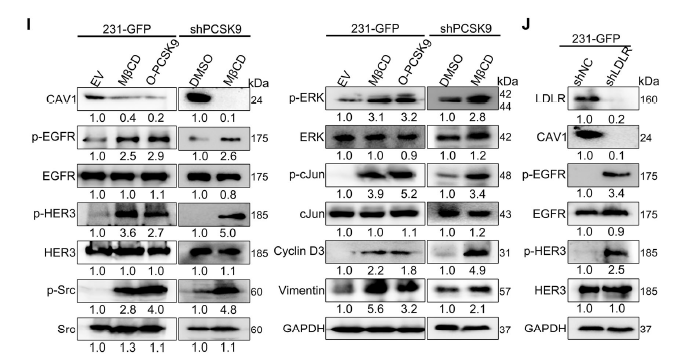
2. Key contributions
- Confirmed stability of upstream signalling molecules: PCSK9 selectively enhances EGFR phosphorylation (p-EGFR) without altering total EGFR levels, substantiating lipid-raft-mediated activation.
- Ensured mechanistic accuracy: High antibody specificity eliminated cross-reactivity, producing crisp EGFR bands essential for densitometric quantification.
3. Result presentation
Figure 5A demonstrates unchanged total EGFR levels in 231-GFP, 4-11 and PCSK9-manipulated cells, whereas p-EGFR fluctuates significantly—direct evidence that PCSK9 activates rather than up-regulates EGFR.
IV. Implications and Absin product value
This study not only unravels a previously unrecognised oncogenic function of PCSK9 in TNBC, but also provides a scientific rationale for developing PCSK9 inhibitors and combined EGFR/HER3-targeted therapies. As a research-tool provider, Absin continuously supports fundamental and translational biomedical science with premium antibodies.
Absin’s EGFR antibody (abs149686) is validated for high purity (SDS-PAGE), high affinity (multi-assay verified) and lot-to-lot consistency, meeting the stringent requirements of signalling-pathway analysis. Its successful deployment in this top-tier publication reaffirms the reliability of Absin reagents in life-science research.
| Product | Cat. No. | Application | Key advantages |
|---|---|---|---|
| Mouse anti-EGFR Monoclonal Antibody | abs149686 | Western Blot, protein expression analysis | High specificity, high stability, batch consistency |
| Note: Validated in multiple studies for reliable detection of key signalling molecules. | |||
This article is based on the original publication in Advanced Science (DOI: 10.1002/advs.202408514). All figures and data cited remain the intellectual property of the original journal and the corresponding authors. If any infringement is identified, please contact us for prompt removal.
Contact Absin
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
| Absin Bioscience Inc. worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
