- Cart 0
- English
Overcoming KRAS-Inhibitor Resistance: Absin Organoid Culture Kit Powers SAT1–Polyamine–Ferroptosis Precision-Oncology Study
December 22, 2025
Clicks:176
In oncology, KRAS-mutant cancers have long remained an intractable clinical challenge due to intrinsic and acquired resistance. Co-occurring KEAP1 mutations further blunt therapeutic efficacy. A breakthrough study published in Nature Communications now unveils a precision-medicine strategy that exploits tumor polyamine metabolism and ferroptosis in a KEAP1-status-dependent manner, markedly potentiating KRAS-targeted therapy. Absin organoid culture reagents were instrumental in validating the proposed mechanism.
Title: Targeting polyamine metabolism and ferroptosis enhances the efficacy of KRAS-targeted therapy depending on KEAP1 status
Journal: Nature Communications (IF 15.7)
DOI: https://doi.org/10.1038/s41467-025-65441-4
Absin reagent: Organotial Human Lung Cancer Organoid Culture Kit (abs9443)
I. Concept: Exploiting Metabolic Vulnerabilities to Overcome Resistance
KRAS mutations drive pancreatic, colorectal, and lung adenocarcinomas. Although KRASG12C inhibitors such as sotorasib are approved, co-occurring KEAP1 mutations confer profound resistance. The authors hypothesized that:
- 1. Tumor cells depend on polyamine metabolism for proliferation; targeting this pathway could sensitize tumors to KRAS inhibition;
- 2. KEAP1 status dictates expression of polyamine-metabolic enzymes and thus therapeutic response;
- 3. Polyamine metabolism intersects with ferroptosis, offering a synergistic anti-cancer modality.
A step-wise pipeline—metabolite library screening → cell/organoid validation → mechanistic dissection → in-vivo confirmation—ultimately defined a KRAS–KEAP1–SAT1–ferroptosis regulatory axis and a genotype-guided treatment paradigm.
II. Key Findings: Genotype-Stratified Therapy Doubles Efficacy
1. Polyamine supplementation sensitizes in a KEAP1-dependent manner
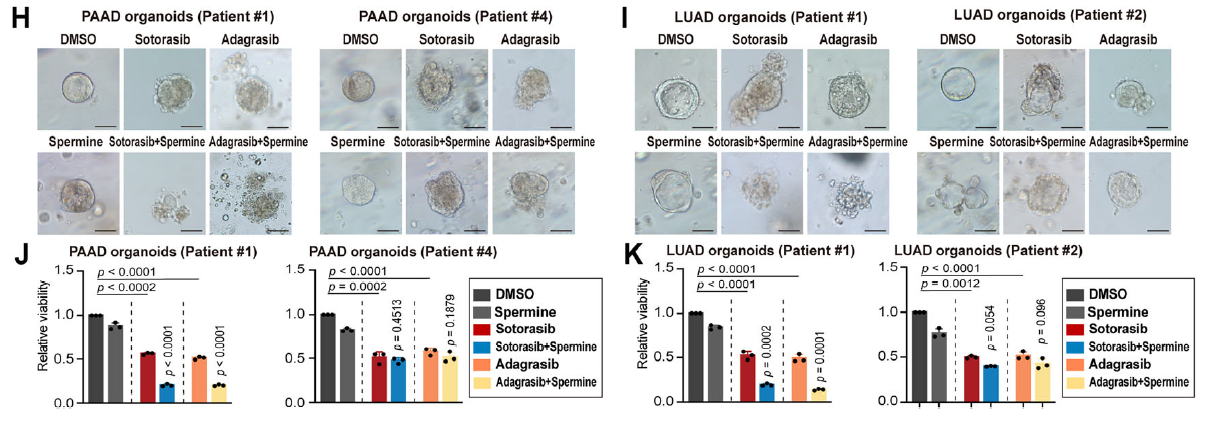
- In KRASMU/KEAP1WT cells, patient-derived organoids (PDOs), and xenografts, exogenous spermidine or spermine markedly enhanced KRAS-inhibitor efficacy (Fig. 1H–K);
- KRASMU/KEAP1MU models required lentivirus/AAV-mediated SAT1 over-expression plus polyamine supplementation to achieve sensitization (Fig. 6C–D).
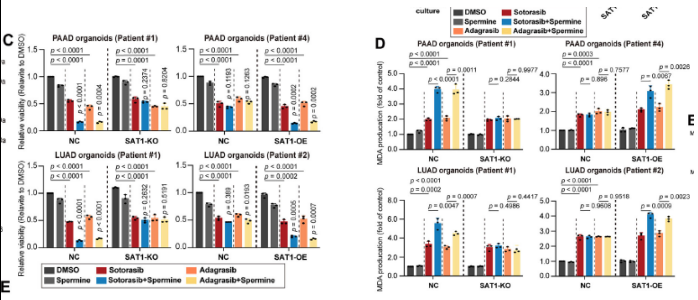
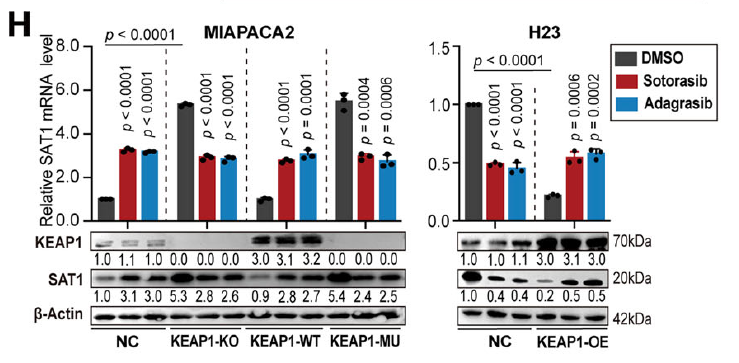
2. SAT1 is the central metabolic node
SAT1 (spermidine/spermine N1-acetyltransferase) is rate-limiting for polyamine catabolism and is regulated by KEAP1 status:
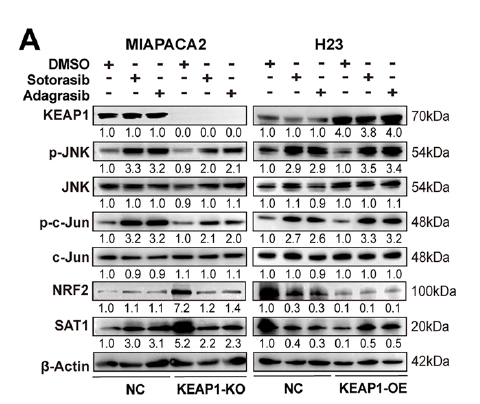
- In KEAP1WT cells, KRAS inhibition activates the JNK/c-Jun axis, up-regulating SAT1, promoting polyamine catabolism, ROS accumulation, and ferroptosis (Fig. 5A);
- In KEAP1MU cells, constitutive NRF2 activation accelerates JNK degradation, repressing SAT1; exogenous SAT1 restoration is required to re-sensitize tumors (Fig. 2H).
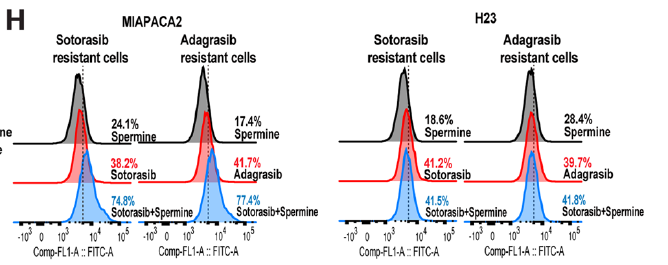
3. Overcoming acquired resistance
Long-term sotorasib-resistant cells remained susceptible to the polyamine–SAT1 regimen, which restored lipid-ROS accumulation and elevated MDA levels, confirming ferroptotic cell death (Fig. 4H) and offering a salvage strategy for resistant disease.
III. Powered by Absin: Organoid Culture Kits as Core Enablers
Absin PDO reagents provided robust, reproducible organoid models for all functional assays:
| Product | Cat# | Application | Key Roles |
|---|---|---|---|
| Organotial Human Lung Cancer Organoid Culture Kit | abs9443 | Establishment of PDOs from KRAS-mutant pancreatic (PAAD) and lung (LUAD) patient tumors (Fig. 1F–G) | 1. Supplies tumor-specific nutrients maintaining 3D architecture & intratumoral heterogeneity; 2. Supports long-term expansion for polyamine + KRAS-inhibitor synergy assays; 3. Enables CRISPR-mediated SAT1 KO/over-expression for functional validation. |
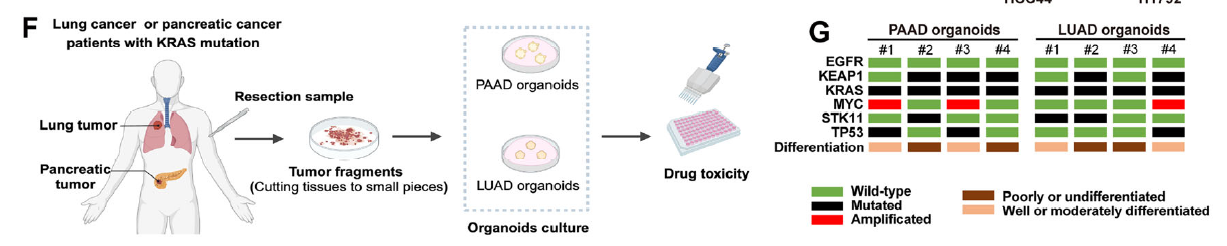
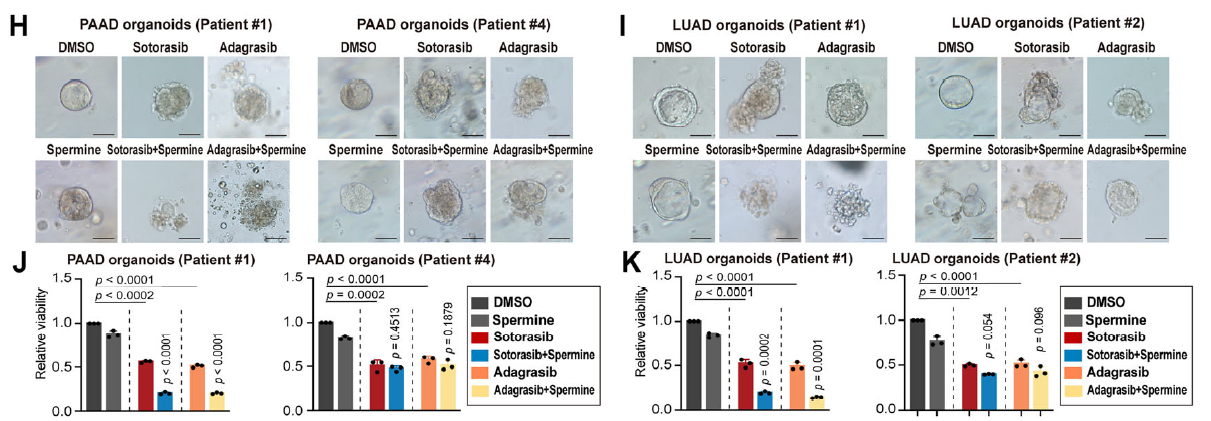
Patient-derived organoids faithfully recapitulate in-vivo tumor biology and have become a cornerstone of precision-oncology research. Absin’s PDO culture kits, recognized for robustness and batch-to-batch consistency, accelerated the clinical translation of the polyamine-sensitization strategy.
IV. Clinical Implications & Future Directions
This study establishes KEAP1 genotype as a decisive biomarker for polyamine-targeted interventions and proposes a stratified regimen: KRAS inhibitor + polyamine supplementation (KEAP1-WT) versus SAT1 over-expression + polyamine supplementation + KRAS inhibitor (KEAP1-mutant), setting a new paradigm for precision oncology in KRAS-driven cancers.
Content summarized from Nature Communications (DOI: 10.1038/s41467-025-65441-4). All original figures and data rights belong to the journal and authors. Please contact us for prompt removal if any infringement is identified.
Contact Absin
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
| Absin Bioscience Inc. worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
