worldwide@absin.cn
- Cart 0
- English
A Comprehensive Protocol for Culturing H9 Embryonic Stem Cells
December 01, 2025
Clicks:77
Reagents & Consumables for the Complete Workflow
| Category | Cat. No. | Product Name | Size |
|---|---|---|---|
| Stem Cells | abs90248 | H9 Human Embryonic Stem Cells, Feeder-Free | 1 mL |
| Pluripotent Stem-Cell Medium | abs90487 | hESC/iPSC Cell-Culture Kit | 500 mL |
| Coating Solution | abs9496 | Matrigel®, iPSC-Qualified, Phenol-Red-Free | 1.5 mL × 4 |
| Basal Medium | abs9560 | DMEM/F-12 Medium | 500 mL |
| Passage Dissociation Reagent (Clumps) | abs90493 | hESC/iPSC Passaging Working Solution, Enzyme-Free | 500 mL |
| DPBS | abs970 | D-PBS Buffer (1×, Ca²⁺/Mg²⁺-free) | 500 mL |
| Stem-Cell Freezing Medium | abs9412 | ES/iPSC Cryopreservation Medium, Serum-Free | 100 mL |
| Cell-Culture Consumables | abs7033 | Cell-Culture Plate, Standard Clear 6-Well | 1 case |
| abs7034 | Cell-Culture Plate, Standard Clear 12-Well | 1 case | |
| abs7035 | Cell-Culture Plate, Standard Clear 24-Well | 1 case | |
| abs7053 | 10 mL Disposable Serological Pipette | 1 case | |
| abs7054 | 25 mL Disposable Serological Pipette | 1 case | |
| abs7164 | 2 mL Internal-Thread Cryovial | 1 case | |
| abs7289 | 2 mL Low-Temperature Metal Ice Box (24-well, flat-bottom) | 1 pc |
I. Key Points for Thawing
1. Preparation of hESC/iPSC Complete Medium
| Product Information | Size | Storage |
|---|---|---|
| hESC/iPSC Basal Medium | 450 mL | 2–8 °C, 12 months |
| hESC/iPSC Growth Supplements A | 50 mL | –20 °C or –80 °C, 12 months |
| hESC/iPSC Supplement C (Y27632) | 1 mL (5 mM) | –20 °C or –80 °C, 12 months |
| Component | 500 mL | 100 mL | 50 mL |
|---|---|---|---|
| hESC/iPSC Basal Medium | 450 mL | 90 mL | 45 mL |
| hESC/iPSC Growth Supplements A | 50 mL | 10 mL | 5 mL |
- Thaw Supplement A overnight at 4 °C; do NOT use 37 °C incubator or water bath. Limit freeze-thaw cycles of A to ≤ 2; aliquot 5 mL per tube.
- Thaw Supplement C overnight at 4 °C; do NOT use 37 °C incubator or water bath. Limit freeze-thaw cycles of C to ≤ 2; aliquot 100 µL per tube. Y27632 is added only on the first day of recovery/passage and for cryopreservation.
- Refer to Table 1: aseptically combine components to prepare hESC/iPSC complete medium; store at 4 °C and use within 2 weeks. Complete medium may be aliquoted and stored at –20 °C for up to 6 months.
- Pre-warm complete medium to room temperature (no longer feels cold) before use.
2. Matrigel® Coating (6-well plate example)
abs9496 – 1.5 mL × 4
- Thaw Matrigel® overnight at 4 °C on ice. Pre-chill 200 µL and 1 mL pipette tips at –20 °C overnight.
- Dilute Matrigel® stock 1:100 with ice-cold DMEM/F-12. Example: add 1.5 mL Matrigel® to 150 mL cold DMEM/F-12; coating density = 300 µL cm⁻². A 6-well plate (10 cm² per well) requires 3 mL per well. Spread evenly to cover the entire surface. Use remaining coating solution within 2 weeks at 4 °C.
- Pre-warm the coated plate in the incubator.
- Incubate the coated plate 1 h at room temperature; prevent the surface from drying.
- Aspirate coating solution and immediately seed stem-cell suspension.
3. Thawing hESC/iPSC (6-well plate example)
- Pre-heat water bath to 37 °C; place Matrigel®-coated 6-well plate in biosafety cabinet ~1 h to equilibrate to room temperature (15–30 °C).
- Add 6 µL Supplement C to 3 mL hESC/iPSC complete medium (final 10 µM). Prepare 9 mL DMEM/F-12 + 18 µL Supplement C (10 µM). Equilibrate both to room temperature (15–30 °C). Do NOT pre-warm media in 37 °C water bath.
- Thaw 1 vial of frozen cells in 37 °C water bath with gentle agitation for ≤ 2 min; remove when only a tiny ice crystal (mung-bean size) remains.
- Wipe vial with 75 % ethanol; transfer to biosafety cabinet. Move cell suspension to a 15 mL conical tube. Drop-wise add 10 mL DMEM/F-12 + Y27 while gently swirling. Centrifuge 160 × g, 5 min.
- Aspirate coating solution from wells. Gently add 2 mL complete medium + Supplement C per well by wall-running.
- Aspirate supernatant leaving ~50 µL. Flick tube 3–4× to resuspend cells. Drop-wise add 1 mL complete medium + Supplement C, flick 3–4×, and seed the 1 mL drop-wise into the 2 mL medium already in the well.
- Mix by horizontal cross-swirling 3× (1 cycle = left-right + up-down). Incubate at 37 °C, 5 % CO₂, saturated humidity; repeat cross-swirling 3× after placement.
- Change to fresh complete medium 18–24 h later, then daily (2 mL per well for 6-well plate). When confluency > 50 %, increase volume to 3–4 mL per well.
II. Key Points for Passaging
1. Criteria & Split Ratios
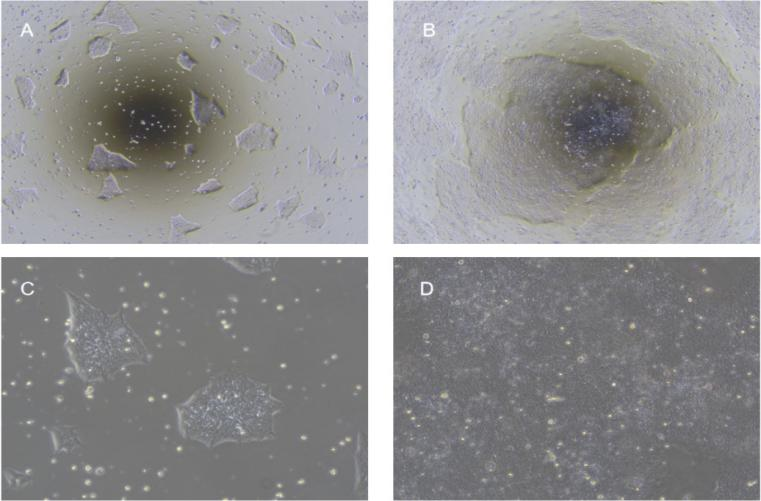
Figure 1. Morphology of hiPSC cultured in hESC/iPSC complete medium: (A, B) low-power view on days 2 & 4; (C, D) high-power view on days 2 & 4.
(1) Passage Criteria:
- Confluency ~85 % (Figure 1C–D); usually passage every 3–4 days;
- Colonies overly dense or large;
- Increased differentiation within colonies.
Note: Do not culture continuously > 5 days even if colonies are small or sub-confluent.
(2) Split Ratio:
Adjust split ratio from 1:5 to 1:12 according to growth status and experimental needs.
- Normal colonies, ~85 % confluency, uniform size (Figure 1B–D): recommended 1:10;
- Lower density → decrease ratio;
- Higher density → increase ratio.
Note: 1:10 split = 1 well into 10 wells (6-well plate example).
2. Dissociation
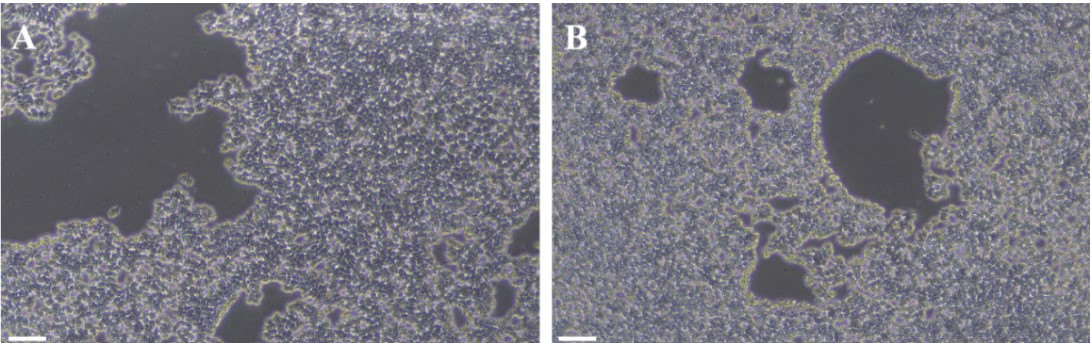
Figure 2. hiPSC morphology during dissociation: (A) 5 min; (B) 6 min.
- Equilibrate Matrigel®-coated 6-well plate, hESC/iPSC Passage Solution, and DPBS to room temperature (~25 °C) in biosafety cabinet for ~1 h.
- Prepare 2 mL + 1 mL complete medium per well to be seeded; add Supplement C to 10 µM and equilibrate to ~25 °C.
- Aspirate spent medium; gently rinse with 1 mL DPBS (Ca²⁺/Mg²⁺-free) per well and aspirate.
- Add 1 mL hESC/iPSC Passage Solution per well to completely cover the surface.
- Incubate at 37 °C for 5–6 min.
Note: ① After 5–6 min inspect under microscope—cells should appear bright and round but still attached. If most cells are not yet bright, extend incubation (total < 10 min). ② Keep plate in direct contact with incubator shelf to ensure even heating; do not stack plates.
- Return plate to biosafety cabinet gently without shaking; tilt and aspirate Passage Solution.
- Immediately add 2 mL pre-warmed complete medium + Supplement C per well. Gently flush once with the medium in the pipette tip, then re-triturate once to detach cells.
Note: ① Limit trituration to 1–2 gentle strokes. 10–15 % cells remaining attached is normal; if majority remain, extend dissociation (< 10 min). ② Work quickly—keep cells in Passage Solution < 15 min and process ≤ 4 wells per batch.
- Aspirate residual Matrigel® solution; add 2 mL pre-warmed complete medium + Supplement C per well.
- Label plate with cell line, passage number, split ratio, date, operator. Mix cell suspension gently and distribute according to predetermined ratio.
- Mix by horizontal cross-swirling 3×; incubate at 37 °C, 5 % CO₂, saturated humidity; repeat cross-swirling 3× after placement.
- Change to fresh complete medium 18–24 h later, then daily. Passage or freeze again after 4–5 days.
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
