- Cart 0
- English
A Comprehensive Protocol for Culturing H1 Human Embryonic Stem Cells
November 21, 2025
Clicks:142
Reagents and Consumables for the Entire Workflow
| Category | Cat. No. | Product Name | Specification |
|---|---|---|---|
| Stem Cells | abs90289 | hESC(H1) Human Embryonic Stem Cells | 1 mL |
| Pluripotent Stem Cell Medium | abs90487 | hESC/iPSC Cell Culture Kit | 500 mL |
| Coating Solution | abs9496 | Matrigel (iPSC-qualified, Phenol Red-free) | 1.5 mL × 4 |
| Basal Medium | abs9560 | DMEM/F-12 Medium | 500 mL |
| Passaging Dissociation Solution (Clumps) | abs90493 | hESC/iPSC Passaging Working Solution (Enzyme-free) | 500 mL |
| DPBS | abs970 | D-PBS Buffer (1×, Ca²⁺/Mg²⁺-free) | 500 mL |
| Stem Cell Freezing Medium | abs9412 | ES/iPS Cell Cryopreservation Medium | 100 mL |
| Cell Consumables | abs7033 | Cell Culture Plate (Standard Clear 6-well) | 1 case |
| abs7034 | Cell Culture Plate (Standard Clear 12-well) | 1 case | |
| abs7035 | Cell Culture Plate (Standard Clear 24-well) | 1 case | |
| abs7053 | 10 mL Disposable Serological Pipette | 1 case | |
| abs7054 | 25 mL Disposable Serological Pipette | 1 case | |
| abs7164 | 2 mL Internal-thread Cryovial | 1 case | |
| abs7289 | 2 mL Low-temperature Metal Ice Box (24-well, flat-bottom) | 1 pc |
I. Key Points for Recovery
1. Preparation of hESC/iPSC Complete Medium
Table 1. hESC/iPSC Cell Culture Kit
| Product Information | Specification | Storage |
|---|---|---|
| hESC/iPSC Basal Medium | 450 mL | 2–8 °C, 12 months |
| hESC/iPSC Growth Supplements A | 50 mL | –20 °C or –80 °C, 12 months |
| hESC/iPSC Supplement C (Y27632) | 1 mL (5 mM) | –20 °C or –80 °C, 12 months |
Table 2. Preparation Instructions for hESC/iPSC Complete Medium
| Component | 500 mL | 100 mL | 50 mL |
|---|---|---|---|
| hESC/iPSC Basal Medium | 450 mL | 90 mL | 45 mL |
| hESC/iPSC Growth Supplements A | 50 mL | 10 mL | 5 mL |
- Thaw Supplement A overnight at 4 °C; do not thaw in 37 °C incubator/water bath. Do not exceed 2 freeze-thaw cycles. Aliquot into 5 mL tubes.
- Thaw Supplement C overnight at 4 °C; do not thaw in 37 °C incubator/water bath. Do not exceed 2 freeze-thaw cycles. Aliquot into 100 µL tubes. Y27632 is used only on the first day of recovery/passaging and for cryopreservation.
- Refer to Table 1, mix the components aseptically to prepare hESC/iPSC complete medium. Store at 4 °C and use within 2 weeks. Complete medium can be aliquoted and stored at –20 °C for up to 6 months.
- Before use, pre-warm complete medium to room temperature until no longer cold to touch.
2. Matrigel Coating (6-well plate example)
Matrigel (iPSC-qualified, Phenol Red-free, abs9496, 1.5 mL × 4, 10 mg/mL, store at –80 °C for 2 years)
- Thaw Matrigel overnight at 4 °C on ice. Pre-chill 200 µL and 1 mL tips at –20 °C overnight.
- Dilute Matrigel stock 1:100 with ice-cold DMEM/F-12. Example: add 1.5 mL Matrigel to 150 mL ice-cold DMEM/F-12. Coating density: 300 µL/cm²; 6-well plate = 10 cm²/well, use 3 mL per well, spread evenly. Use remaining coating solution within 2 weeks at 4 °C.
- Pre-warm coated plates in incubator.
- Let coated plate sit at room temperature for 1 h; do not allow surface to dry.
- Aspirate coating solution and immediately seed stem-cell suspension.
3. Recovery of hESC/iPSC (6-well plate example)
- Pre-heat water bath to 37 °C; place Matrigel-coated 6-well plate in biosafety cabinet for ~1 h to equilibrate to room temperature (15–30 °C).
- Add 6 µL Supplement C to 3 mL hESC/iPSC complete medium (final 10 µM); add 18 µL Supplement C to 9 mL DMEM/F-12 (final 10 µM); bring both to room temperature (15–30 °C). Do not pre-warm media in 37 °C water bath.
- Thaw 1 vial of frozen cells in 37 °C water bath with gentle agitation for ≤2 min; remove when ice crystals are almost gone (pea-size).
- Wipe vial with 75 % ethanol; transfer to biosafety cabinet; pipette cell suspension into 15 mL tube, add 10 mL Y27-containing DMEM/F-12 drop-wise with gentle mixing, centrifuge 160 ×g for 5 min.
- Aspirate coating solution from wells, gently add 2 mL hESC/iPSC complete medium + Supplement C per well.
- Aspirate supernatant leaving ~50 µL, flick tube 3–4× to resuspend, add 1 mL complete medium + Supplement C drop-wise, flick again, seed the 1 mL suspension into the 2 mL medium in well.
- Cross-shake plate 3× (left-right + up-down = 1×), incubate at 37 °C, 5 % CO₂, saturated humidity; repeat cross-shake once more.
- Change to fresh hESC/iPSC complete medium after 18–24 h, then daily (2 mL/well). If confluency >50 %, increase volume to 3–4 mL/well.
II. Key Points for Passaging
1. Conditions and Ratio
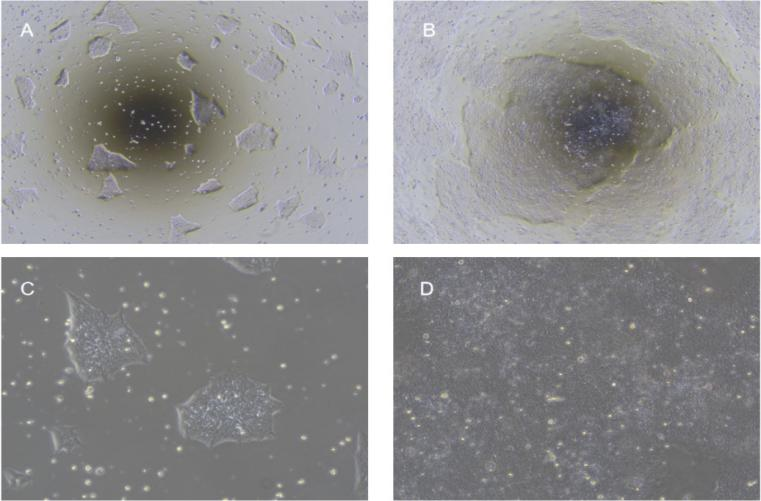
Fig. 1. Morphology of hiPSC cultured in hESC/iPSC complete medium: (A, B) low-power view on days 2 and 4; (C, D) high-power view on days 2 and 4.
(1) Passaging conditions:
- Cells reach ~85 % confluency (Fig. 1C–D), usually every 3–4 days.
- Colonies overly dense or large.
- Increased differentiation observed.
Note: Do not continuously culture >5 days even if colonies are small.
(2) Splitting ratio:
- Generally 1:5–1:12 according to growth status and experimental needs.
- If 85 % confluency and uniform colonies (Fig. 1B–D), use 1:10.
- Reduce ratio if density is low; increase if high.
Note: 1:10 means 1 well → 10 wells (6-well plate).
2. Dissociation

Fig. 2. hiPSC morphology during dissociation: (A) 5 min; (B) 6 min.
- Equilibrate Matrigel-coated 6-well plate, hESC/iPSC Passage Solution, and DPBS to room temperature (~25 °C) in biosafety cabinet for ~1 h.
- Prepare hESC/iPSC complete medium + Supplement C (10 µM) at 2 mL/well + 1 mL extra, bring to ~25 °C.
- Aspirate spent medium, rinse with 1 mL/well Ca²⁺/Mg²⁺-free DPBS, aspirate.
- Add 1 mL/well hESC/iPSC Passage Solution to completely cover surface.
- Incubate at 37 °C for 5–6 min.
① Observe under microscope: most cells should become bright and rounded but still attached; extend time if needed, but <10 min total.
② Keep plate in direct contact with metal shelf, do not stack.
- Return plate to biosafety cabinet without shaking, tilt and aspirate Passage Solution.
- Immediately add 2 mL/well pre-warmed complete medium + Supplement C, gently pipette once to detach cells; collect and dispense once more.
① Limit to 1–2 gentle pipettings; 10–15 % cells remaining is normal. Extend dissociation if majority remain attached (<10 min).
② Work quickly, process ≤4 wells at a time to avoid prolonged exposure (<15 min).
- Aspirate Matrigel solution from new plate, add 2 mL/well pre-warmed complete medium + Supplement C.
- Label plate with cell line, passage number, split ratio, date, operator. Distribute cell suspension evenly according to desired ratio.
- Cross-shake 3×, incubate at 37 °C, 5 % CO₂, saturated humidity; repeat cross-shake once more.
- Change to fresh complete medium after 18–24 h, then daily. Passage or freeze 4–5 days later.
Absin’s Pick of the Week
| Cat. No. | Product Name | Specification |
|---|---|---|
| abs90289 | hESC(H1) Human Embryonic Stem Cells | 1 mL |
| abs90487 | hESC/iPSC Cell Culture Kit | 500 mL |
| abs9496 | Matrigel (iPSC-qualified, Phenol Red-free) | 1.5 mL × 4 |
| abs9560 | DMEM/F-12 Medium | 500 mL |
| abs90493 | hESC/iPSC Passaging Working Solution (Enzyme-free) | 500 mL |
| abs970 | D-PBS Buffer (1×, Ca²⁺/Mg²⁺-free) | 500 mL |
| abs9412 | ES/iPS Cell Cryopreservation Medium | 100 mL |
| abs7033 | Cell Culture Plate (Standard Clear 6-well) | 1 case |
| abs7034 | Cell Culture Plate (Standard Clear 12-well) | 1 case |
| abs7035 | Cell Culture Plate (Standard Clear 24-well) | 1 case |
| abs7053 | 10 mL Disposable Serological Pipette | 1 case |
| abs7054 | 25 mL Disposable Serological Pipette | 1 case |
| abs7164 | 2 mL Internal-thread Cryovial | 1 case |
| abs7289 | 2 mL Low-temperature Metal Ice Box (24-well, flat-bottom) | 1 pc |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
