- Cart 0
- English
Cell Therapy Goes Full-Drug: End-to-End Deconstruction of In Vivo CAR-T
November 14, 2025
Clicks:159
Abstract
CART technology is rapidly evolving from conventional “ex vivo programming” to “in vivo direct reprogramming”. The former has established efficacy and standardized workflows in hematologic malignancies, yet suffers from manufacturing complexity, high cost and limited accessibility. The latter relies on tropism-modified viral vectors or nano-formulated nucleic acids (mRNA/DNA) to engineer immune cells directly in patients, promising streamlined logistics, lower cost and improved safety/biological profiles. Here we systematically compare the two technological routes, key manufacturing steps, core differences and application scenarios based on cutting-edge reviews and translational data, providing a reference for R&D and clinical translation teams.
I. What is CAR-T Cell Therapy?
CAR-T therapy refers to the ex vivo genetic engineering of patient-derived peripheral-blood T cells to express a chimeric antigen receptor (CAR) that specifically recognizes tumor-associated antigens. After activation and expansion in vitro, the CAR-T product is re-infused to eradicate malignant cells.
Since the first CAR-T approvals in 2017, the global market has expanded continuously: sales reached USD 2.014 billion in H1-2024 and are projected to hit USD 21.8 billion by 2030.
CAR architecture has undergone five generational iterations. All five generations share four conserved domains: extracellular antigen-binding domain, hinge, trans-membrane domain and intracellular signalling domain.
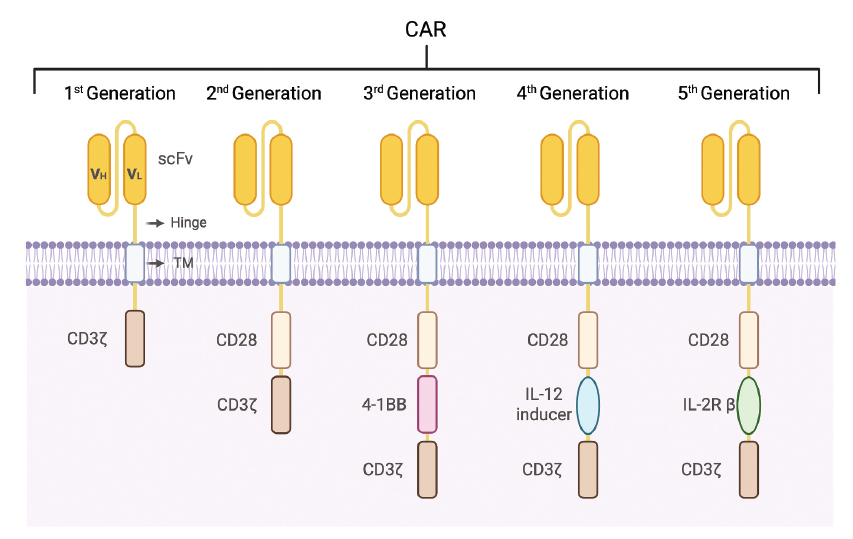
Evolution of CAR structure [1]
Functional comparison is summarized below:
| Generation | Core structural hallmark | Functional advantage |
|---|---|---|
| 1st | CD3ζ only; no co-stimulatory domain | Basic antigen recognition & T-cell activation; simplest structure |
| 2nd | CD3ζ + 1 co-stimulatory domain (CD28 or 4-1BB) | Amplified activation signal; enhanced proliferation & persistence |
| 3rd | CD3ζ + 2 distinct co-stimulatory domains (CD28-4-1BB or ICOS-4-1BB) | Further strengthened signalling; superior cytotoxicity & durability versus 2nd |
| 4th | CD3ζ, co-stimulatory domain + transgenic cytokine (e.g. IL-12, IL-15) | Activation-triggered release of immunostimulatory cytokines; augmented tumour infiltration |
| 5th | TCR-like activation, co-stimulatory & cytokine signalling in one universal CAR | Near-physiological cytokine signalling; comprehensive T-cell potentiation |
II. End-to-End Roadmap of Ex vivo and In vivo CAR-T Pathways
1. Conventional Ex vivo CAR-T
- Patient screening & referral → Leukapheresis → T-cell activation (CD3/CD28, cytokines) → Gene transfer (lentiviral/γ-retroviral or non-viral) → Ex vivo expansion & QC release → Lymphodepletion (cyclophosphamide/fludarabine) → Infusion & monitoring
Pain Points & Bottlenecks
- Manufacturing complexity and long vein-to-vein time (2–7 weeks); bridging therapy often required for rapidly progressive disease
- Patient-specific products → batch-to-batch variability, unstable yields, high cost
- Lymphodepletion causes prolonged leukopenia and infection risk
- Entire workflow demands high GMP capacity, cold-chain logistics and multi-site coordination
2. In vivo CAR-T
- A single “off-the-shelf” formulation is injected directly into the patient; host T cells are genetically re-programmed in situ to express CAR and expand into functional cytotoxic populations
Major Implementation Routes
- i. Targeted viral vectors: engineered lentivirus/retrovirus or AAV whose envelope/capsid is pseudotyped and decorated with T-cell–directed ligands (scFv, nanobody, DARPin) to selectively transduce CD3/CD4/CD8 subsets
- ii. Non-viral nano-delivery: lipid nanoparticles (LNP) or biodegradable polymeric nanoparticles (e.g., PBAE) encapsulating mRNA or DNA; surface conjugation with anti-CD3/CD4/CD5/CD8 or lipid-composition tuning enhances T-cell uptake and expression
Key Attributes
- i. Scalable, standardized “one-product” inventory with immediate availability
- ii. Lymphodepletion can be omitted or minimized, preserving immune networks and favouring epitope spreading
- iii. mRNA formats give transient expression, allowing programmable repeat-dosing/dose-titration that balances efficacy and safety
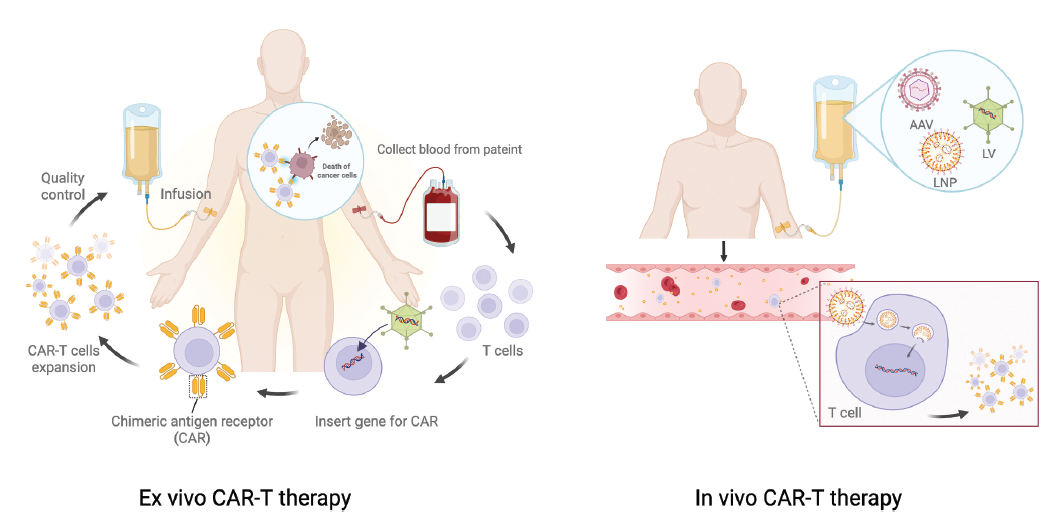
Schematic of ex vivo and in vivo CAR-T workflows [1]
III. Technical Essentials for In vivo CAR-T
1. Vector/Delivery System & Targeting Strategy
Viral Vectors
- Lentivirus: pseudotyped envelopes (VSV-G, measles, NiV, Sindbis, cocal, etc.) plus T-cell-targeting ligands (anti-CD3/CD4/CD8 scFv/DARPin) enable selective tropism and membrane fusion; integrase activity supports durable CAR expression
- AAV: capsid surface-display of targeting ligands; episomal persistence provides medium-term expression without integration
Non-viral Nano-delivery
- LNP: ionizable cationic lipid + helper lipids (phospholipid/cholesterol/PEG-lipid); endosomal escape releases mRNA; antibody-conjugation further enhances T-cell uptake
- Polymeric NPs: deliver mRNA or DNA; peptide/antibody decoration improves specificity; can be combined with transposon systems for prolonged expression (safety liabilities require careful assessment)
- Exosomes: engineered exosomes (e.g., dendritic-cell-derived) surface-armed with anti-CD3 or anti-EGFR nanobodies
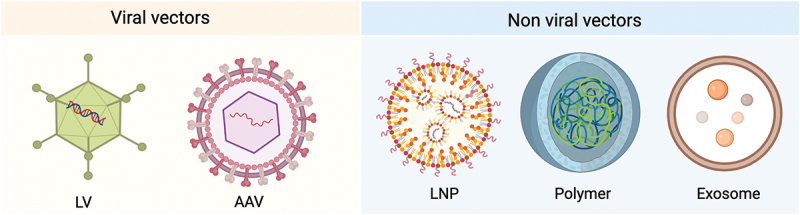
Delivery vectors
2. Nucleic-acid Construct & Expression Kinetics
- mRNA design: cap analogues, optimized UTRs (α-/β-globin 5′/3′UTRs), N1-methyl-pseudouridine, poly(A) tail length collectively enhance stability, translation and reduce innate sensing
- Co-delivery: mRNA can be packaged together with siRNA (e.g., PD-1 siRNA) to synchronously relieve immune suppression
- Expression window: mRNA-LNP gives transient expression (days), amenable to “repeat-dose → real-time dose/toxicity tuning → avoidance of exhaustion”; DNA/integrating platforms prolong expression but raise insertional mutagenesis and off-target transduction concerns
3. Specificity & Safety
Consequences of Off-target Transduction/Transfection
- Transduction of tumour cells → “epitope masking” (cis-binding renders antigen inaccessible)
- Transduction of suppressive immune subsets (e.g., Treg) may exacerbate disease
- Germ-line or stem-cell transduction (viral integration) poses oncogenic or heritable risks
Immunogenicity & Repeat Dosing
- Intrinsic immunostimulatory properties of LNP lipids and complement activation require management (pre-medication protocols validated for marketed LNP-siRNA can be adapted)
- Pre-existing neutralising antibodies to AAV or pseudotyped envelopes
- Balancing immune responses against mRNA-LNP during multi-dose regimens
4. Process & Dosing
- Route of administration: intravenous preferred; loco-regional or intracavitary injection considered for selected indications to increase local exposure
- Dose–exposure relationship: mRNA payload, LNP composition (lipid ratio/particle size/surface chemistry), UTR selection and ligand density collectively determine in-vivo T-cell transduction efficiency and expression durability
- Combination strategies: multi-target (parallel mRNAs or sequential vectors), inducible switches, or synthetic cytokine receptors
IV. Ex vivo vs In vivo CAR-T: Key Differences, Pros & Cons
Development/Manufacturing Model
- Ex vivo: patient-specific, complex, long lead-time, high cost, but product attributes are tightly controlled
- In vivo: single batch-scale formulation, greatly simplified manufacturing & release, rapid accessibility
Immunobiology
- Ex vivo: requires lymphodepletion to favour engraftment and expansion; ex-vivo culture may reduce stemness and drive exhaustion
- In vivo: preserves intact immune microenvironment, facilitates epitope spreading; transient mRNA + fractionated dosing mitigates continuous stimulation, lowers CRS/ICANS risk and enables “dose-to-effect” titration
Expression Persistence
- Ex vivo: integrating vector + expansion → long-term persistence
- In vivo: mRNA requires repeat dosing; integrating viruses can be long-term but demand higher specificity and genomic safety margins
Safety Focus
- Ex vivo: monitor insertional mutagenesis, clonal outgrowth, vector integration sites
- In vivo: additional concerns—off-target/germ-line transduction, tumour-cell epitope masking, systemic immunogenicity/anaphylaxis
Indications & Clinical Scenarios
- Ex vivo: established in B-cell malignancies
- In vivo: attractive for disorders requiring broad access and rapid availability (auto-immunity, infection, fibrosis); in oncology can serve as rapid bridging or complement to ex-vivo products
V. Application Landscape & Strategic Recommendations
- Haematological tumours: choose integrating virus or multi-dose mRNA based on relapse risk and depth of remission required
- Autoimmune diseases: CD19/BCMA B-cell depletion; mRNA-LNP preferable for “low-toxicity + adjustable repeat dosing” chronic management
- Infectious diseases (e.g., HIV, HBV): large-scale accessibility and avoidance of lymphodepletion are major advantages
- Fibrosis/tissue repair: transient FAP-CAR or similar for local immune-remodelling in heart failure or liver fibrosis
VI. Translational & Regulatory Considerations
- Viral vectors: demonstrate selective tropism, long-term follow-up of insertion sites/clonal expansion, mitigate germ-line risk
- Non-viral: control formulation consistency, dsRNA residuals, immunogenicity assessment, repeat-dose strategy
- CMC: scalable, batch-consistent processes; critical quality attributes (size/zeta-potential/encapsulation efficiency/ligand stability) must be quantified
- Companion diagnostics: track CAR-cell kinetics in blood/tissue, monitor B-cell/tumour burden, cytokine biomarkers (CRS/ICANS risk)
VII. Absin Products & Services
1. mRNA Synthesis Reagents
| Cat. # | Product Name | Size |
|---|---|---|
| abs60690 | High-Yield T7 Co-transcription RNA Kit (N1-Me-pUTP) | 50 rxn |
| abs60152 | Vaccinia Capping Enzyme | 500/2 k/10 k U |
| abs60153 | T7 RNA Polymerase | 100/250 k U |
| abs60166 | 2´-O-Methyltransferase | 2.5/10/50 k U |
| abs60539 | DNase I | 1/5 k U |
| abs60150 | RNase Inhibitor | 250 µL/1 mL |
Reference: 1. Huang Y, Cao R, Wang S, Chen X, Ping Y, Zhang Y. In vivo CAR-T cell therapy: New breakthroughs for cell-based tumor immunotherapy. Hum Vaccin Immunother. 2025 Dec;21(1):2558403.
Contact Absin
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
| Absin Bioscience Inc. worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
