- Cart 0
- English
Absin Human Liver Cancer Organoid Kit Empowers Precision Medicine to Conquer TKI Resistance
October 21, 2025
Clicks:98
A breakthrough study on the resistance mechanism of targeted therapy in hepatocellular carcinoma (HCC) was recently published online in the top-tier journal Nature Cancer (Impact Factor 28.5). The study unveils that RIOK1 drives tyrosine-kinase inhibitor (TKI) resistance by undergoing liquid–liquid phase separation (LLPS) to nucleate stress granules, sequestering PTEN mRNA and suppressing its translation, thereby activating the pentose-phosphate pathway (PPP) and promoting tumor survival under therapeutic stress. Notably, all patient-derived HCC organoid (PDO) cultures used for translational validation were established with the Absin Human HCC Organoid Kit (abs9444), providing the essential pre-clinical model that underpins the clinical relevance of the findings.
Title: RIOK1 phase separation restricts PTEN translation via stress granules activating tumor growth in hepatocellular carcinoma
Journal: Nature Cancer (IF 28.5)
DOI: https://doi.org/10.1038/s43018-025-00984-5
Key reagent: Organotial Human HCC Organoid Culture Kit (abs9444)


1. Background: Overcoming TKI Resistance in HCC
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related mortality worldwide. Multi-kinase tyrosine-kinase inhibitors (TKIs) such as sorafenib and donafenib remain first-line systemic therapies for advanced disease. However, adaptive cellular reprogramming triggered by tumor-microenvironmental stressors (e.g., nutrient deprivation, hypoxia) frequently culminates in TKI resistance. Although stress granules (SGs) formed by liquid–liquid phase separation (LLPS) have been implicated in chemo-resistance, the precise molecular underpinnings in HCC have remained elusive—an open question that the present study addresses.
2. Key Findings: RIOK1–SG–PTEN Axis as a TKI-Resilience Hub
Multi-platform screening identified the atypical kinase RIOK1 as frequently up-regulated in HCC and associated with poor prognosis. Upon NRF2-mediated transcriptional induction under stress, RIOK1 undergoes LLPS and recruits IGF2BP1 and G3BP1 to nucleate SGs that sequester PTEN mRNA, attenuating its translation. PTEN loss hyper-activates the pentose-phosphate pathway (PPP), bolstering antioxidant capacity and TKI resistance. Pharmacological degradation of RIOK1 by chidamide cooperated with TKIs to suppress tumor growth (Fig. 1).
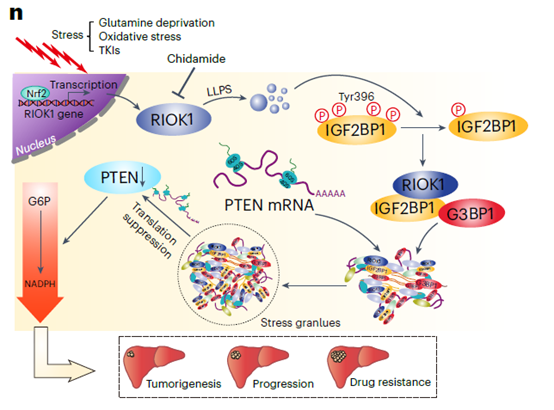
Fig. 1 Schematic of the RIOK1-mediated TKI-resistance pathway
3. Absin Human HCC Organoid Kit: The Translational Linchpin
To verify that the chidamide + donafenib combination is clinically meaningful, the authors generated patient-derived HCC organoids (PDOs) that faithfully preserve intra-tumoral heterogeneity. All PDO cultures were established with the Absin Human HCC Organoid Kit (abs9444), whose contribution is three-fold:
1. Efficient generation of clinically relevant PDOs
Fresh tumor fragments (~1 mm³) from surgically resected HCC (Patients 2 and 7) were enzymatically digested, filtered, and embedded in Matrigel with the kit’s optimized basal medium. Medium was refreshed every 2–3 days; passaging every 5–7 days yielded expandable PDOs (≥P3) exhibiting native histology and mutational profiles.
2. Validation of combination efficacy
PDOs were treated with vehicle, donafenib, chidamide, or the combination. Compared with monotherapies, the combination significantly reduced organoid size (Fig. 2o) and cross-sectional area (Fig. 2p; P < 0.0001) across multiple patient lines, demonstrating broad clinical potential.

Fig. 2 Combination therapy suppresses PDO growth: morphology (left) and quantification (right)
3. Closing the translational loop
H&E staining confirmed that Absin-generated PDOs retained the histo-architecture of parental tumors. Parallel patient-derived xenograft (PDX) studies recapitulated PDO responses, forging an integrated “cellular–organoid–animal” evidence chain in which the Absin kit serves as the indispensable bridge between mechanistic discovery and clinical application.
4. Why Absin Kit Became the Team’s First Choice
The investigators selected the Absin Human HCC Organoid Kit for three decisive reasons:
- High success rate & stability: >80 % of patient samples yield expandable PDOs that maintain heterogeneity for ≥10 passages.
- Clinical fidelity: PDOs faithfully mirror individual patient drug responses, enabling precision-oncology studies.
- User-friendly: ready-to-use complete medium, Matrigel, digestion buffer and SOPs eliminate time-consuming in-house optimization.
5. Conclusion: From Bench to Bedside with Absin
This Nature Cancer paper not only deciphers a previously unrecognized TKI-resistance axis but also underscores the irreplaceable value of PDOs in translational oncology. The reliability and clinical relevance of the Absin Human HCC Organoid Kit (abs9444) have now been validated by a top-tier discovery. Absin will continue to provide one-stop solutions—from reagents to protocols—empowering researchers to accelerate mechanistic insights, drug screening and personalized therapy development.
Need the detailed PDO protocol or more organoid tools? Contact Absin technical support for customized research solutions: 021-38015121.
Products cited
| Cat. # | Product | Size |
|---|---|---|
| abs9444 | Organotial Human HCC Organoid Culture Kit | 1 kit |
More organoid kits
| Tissue | Cat. # | Kit | Size |
|---|---|---|---|
| Liver | abs9544 | Organotial Human Normal Liver Organoid Kit | 1 kit |
| abs9786 | Organotial Human Intrahepatic Cholangiocarcinoma Organoid Kit | 1 kit |
Contact Absin
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
| Absin Bioscience Inc. worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
