- Cart 0
- English
Exosome Research Solutions
September 22, 2025
Clicks:129
Background Introduction
exosomes are a kind of vesicles secreted from cells to the outside of the cell. They were first discovered and named in sheep reticulocytes by Pan and Johnstone. With the deepening of research, people have found that almost all cells, including blood cells, immune cells, cancer cells, stem cells, etc., can produce exosomes. The produced exosomes exist not only in cell culture fluid, but also in blood, urine, breast milk, saliva, pleural effusion and other body fluids. Exosomes can carry some substances in donor cells, and are considered to be important carriers for intercellular communication and material exchange, and can regulate the function and biological behavior of cells directly or indirectly. Studies have shown that exosomes are involved in the development of tumor diseases and can be used as important markers in non-invasive liquid biopsy of tumors. The application of exosomes is mainly in three major fields: disease treatment, biomarkers, and drug delivery.
1. Extraction and purification of exosomes
How to enrich exosomes efficiently and with high purity is the key to realize its omics analysis and functional research. However, exosomes are small in size, low in density, and there is heterogeneity in particle size and composition. Even exosomes produced by the same cell may have different shapes, which brings great difficulties to the extraction of exosomes. Nowadays, many extraction methods based on exosome size, density and immunophenotype have been developed, including ultracentrifugation, density gradient centrifugation, polymer precipitation, ultrafiltration, size exclusion chromatography, immunoaffinity, etc. [1].
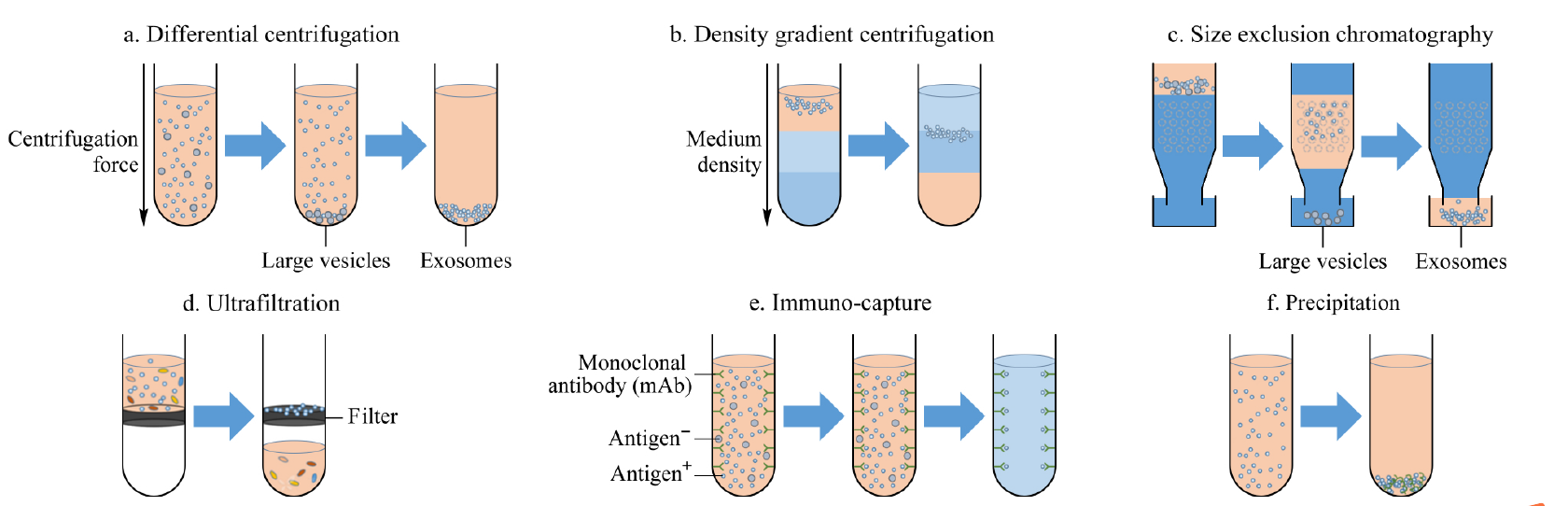
Figure 1 Commonly used exosome isolation methods
- Ultracentrifugation method: First, the cells, cell debris and large vesicles in the sample were removed under the centrifugal force of 300, 2000 and 10000 g, then the exosomes were collected under the condition of 110000 g, and finally the precipitate was washed with phosphate buffer (PBS, abs962) to remove soluble interfering proteins (Figure 1a);
- Density gradient centrifugation method: It is a more stringent ultracentrifugation method, which can further purify vesicles according to the sample density and overcome the pollution caused by protein co-precipitation. Commonly used separation media include iodixanol (abs44121314) and sucrose (abs47050864). Taking sucrose as an example, in actual operation, it is necessary to prepare sucrose/D2O (abs47051532) solutions with different concentration gradients in advance, and then add the substance to be separated. Under the action of ultracentrifugal force, exosomes will reside in the equal density region (1.13 ~ 1.19 g/mL), and purified exosomes can be obtained by collecting this component (Figure 1b);
- Size exclusion chromatography: Polymer gel fillers with different particle sizes are used as separation media to achieve separation of exosomes based on size characteristics (Figure 1c). The column is packed with stable, porous gel particles. When the solution containing exosomes is added to the column, the smaller particles will be trapped in the wells, while the larger molecules will not enter the wells and are eluted first. Thus, the eluted fraction at different stages will contain molecules of different sizes, first larger exosomal particles, followed by smaller protein and lipid particles (abs9777);
- Ultrafiltration method: Its principle is the same as the traditional membrane separation method. This method usually uses an ultrafiltration tube with a relative molecular retention of 100kDa, which can concentrate the sample size while extracting exosomes, and is especially suitable for large-volume samples such as cell culture fluid and urine (Figure 1d);
- Affinity method: Affinity extraction method is currently the most specific exosome extraction method, and it is also the only method that can identify exosomes from specific cells. Affinity extraction of exosomes is mainly achieved by specific interactions between antibodies and exosomal membrane proteins (Fig. 1e). The antibody used is a monoclonal antibody or a polyclonal antibody, and the targetprotein may be a transmembrane protein such as CD63 (abs159125), CD9 (abs131217), and CD81 (abs159270) commonly found on the surface of exosomes, an annexin, an epithelial cell adhesion molecule (EpCAM), or the like, or may be a marker protein unique to the surface of cancer cell exosomes;
- Polymer-based co-precipitation method: Polymerprecipitation method is a strategy commonly used in commercial exosome extraction kits, among which polyethylene glycol (abs42155612) is currently the most commonly used precipitation reagent. It is generally believed that the principle of polymer precipitation method is that the polymer reduces the solubility of exosomes by "hijacking" water molecules, and then sediments under low-speed centrifugation conditions (Figure 1f).
2. Characterization methods of exosomes
At present, exosomes can be characterized by electron microscopy, dynamic light scattering, nanoparticle tracking analyzer, western blotting, enzyme-linked immunosorbent assay, flow cytometry, etc.
The morphological information of exosomes can be obtained by electron microscope. Different types of microscopes have different morphologies of exosomes due to different operating environments. For example, in scanning electron microscopy, transmission electron microscopy, and atomic force microscopy, exosomes exhibit a saucer shape unique to vesicles (Fig. 2a), which may be caused by the extreme dehydration of the sample during fixation or staining, resulting in exosome collapse. In contrast, the exosomes appear round in cryo-electron microscope, and since they will not be dehydrated and denatured, the morphology of the resulting exosomes may be closer to the true state of the vesicles (Fig. 2b). In addition, due to the high resolution of transmission electron microscopy and atomic force microscopy, they can also provide information such as exosome phospholipid bilayer thickness and particle size distribution (Figure 2c).
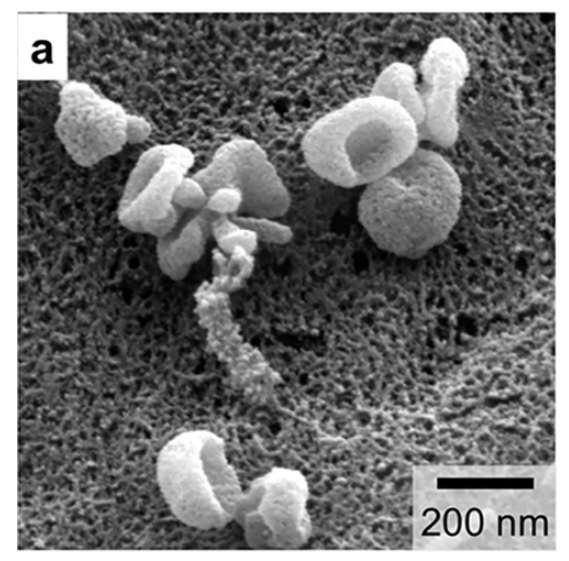
Fig. 2a Under scanning electron microscope, exosomes show a saucer shape unique to vesicles [2].
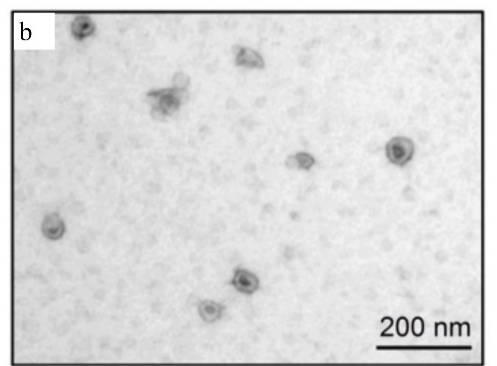
Fig. 2b Exosomes appear round under cryo-electron microscope, and their morphology may be closer to the true state of vesicles [3].
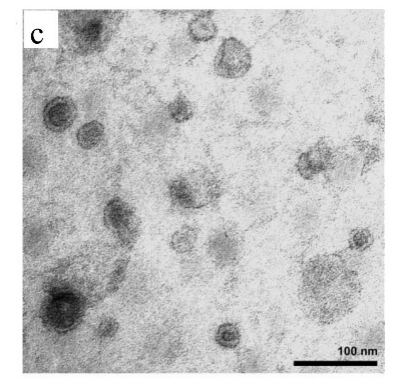
Although several commonly used characterization methods are still insufficient, the combination of multiple methods can improve the characterization accuracy of exosomes. With the development of various technologies, it is expected to achieve accurate, rapid and reliable analysis of exosomes in the future.
3. Exosome tracing technology
In vitro labeling or in vivo tracing of isolated exosomes is helpful to further study the function of exosomes. At present, there are many labeling methods for exosomes, such as fluorescent labeling, biotin labeling, quantum dot labeling, immune labeling, isotope labeling, gene labeling, etc. Among them, fluorescent labeling is the most commonly used exosome labeling method by scientific researchers because of its simple and convenient operation. Exosome-labeled fluorescent dyes include DiR (abs45153692), DiI (abs42002237), DiO (abs45153674), DiD (abs47048165), etc. Among them, the near-infrared fluorescence of DiR can penetrate cells and tissues, and is also commonly used for tracing in small animals. In addition, PKH26 (abs9819) and PKH67 (abs9820) kits for labeling cell membranes were also used for labeling exosomes.
Below, Xiaoai takes the literature as an example to introduce in detail the specific steps of DiR labeling exosomes and using them for in vivo tracing [5].
1)Prepare the separated and purified exosomes;
2)Exosomes were labeledwith 2 μM DiR (abs45153692) at 37 °C for 1 h and then purified with a SuperEV 0.5 purification column.
3)Different amounts of exosomes were intraperitoneally injected into mice. Twenty-four h after injection, mice were anesthetized and imaged using the NIR-II Imaging System H imaging system.
To further analyze the location of exosomes in tissues, tissues were screened, fixed, and sectioned, and the DiR signal was detected using a Nikon TI2-E+A1R confocal microscope (Ex/Em = 748/780 nm).
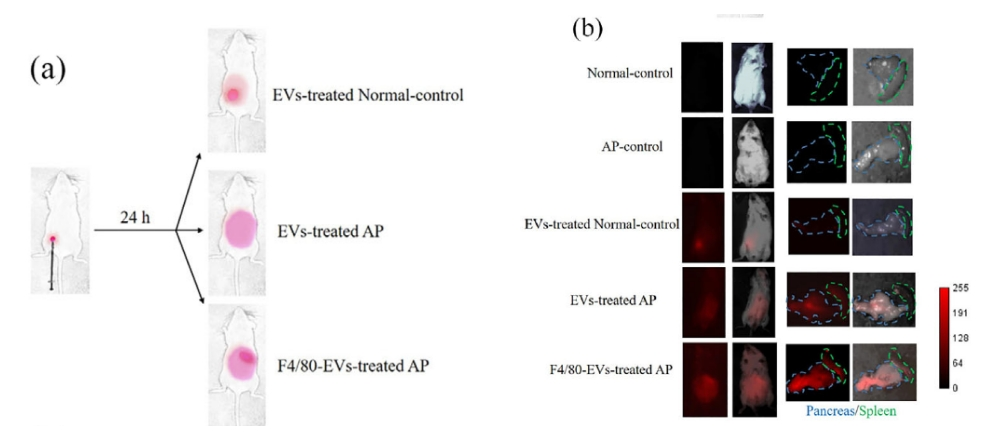
Figure 3 Most fluorescent signals remain at the injection site in normal mice after 24 h treatment with exosomes (EVs) (a). In contrast, in AP mice treated with EVs, the fluorescent signal spread to the abdomen and the pancreas showed a stronger fluorescent signal than in AP mice treated with F4/80-EVs.
Absin can provide researchers with very comprehensive dyes related to cell structure (such as cell membrane, cytoplasm, nucleus, cytoskeleton, mitochondria, lysosomes, exosomes, endoplasmic reticulum, etc.) staining. Regarding the research of exosomes, Xiao Ai will introduce it here today. Welcome all scientific research partners to contact us to discuss experimental issues!
References
[1] Gao Fangyuan, Jiao Fenglong, Zhang Yangjun, et al. Research progress of exosome separation technology and its clinical application [J]. Chromatography, 2019, 37 (10): 13.
[2] Shao H, Im H, Castro C M, et al. New Technologies for Analysis of Extracellular Vesicles [J]. Chemical Reviews, 2018, 118 (4): 1917.
[3] Li P, Kaslan M, Lee S H, et al. Progress in Exosome Isolation Techniques [J]. Theranostics, 2017, 7 (3): 789.
[4] Pisitkun T, Shen R F, Knepper M A. Identification and proteomic profiling of exosomes in human urine [J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101 (36): 13368.
[5] Gao Y, Mi N, Wu W, et al. Transfer of inflammatory mitochondria via extracellular vesicles from M1 macrophages induces ferroptosis of pancreatic beta cells in acute pancreatitis [J]. J Extracell Vesicles, 2024; 13 (2): e12410.
Recommended products for exosome research:
|
Category |
Item number |
Product Name |
|
Exosome Extraction and Purification |
abs42155612 |
Polyethylene glycol |
|
abs44121314 |
Iodixanol |
|
|
abs47050864 |
sucrose |
|
|
abs47051532 |
Heavy water (D2O) |
|
|
abs50039 |
Plasma Serum Exosome Extraction Kit |
|
|
abs50040 |
Cell supernatant exosome extraction kit |
|
|
abs60263 |
Exosome RNA Extraction Kit |
|
|
abs60364 |
Exosome DNA Extraction Kit |
|
|
abs962 |
PBS |
|
|
abs9777 |
Exosome Purification Kit (SEC) |
|
|
Exosome Identification Antibody |
abs131217 |
Rabbit anti-CD9 Recombinant Monoclonal Antibody (143-53) |
|
abs159125 |
Rabbit anti-CD63 Monoclonal Antibody (4G3) |
|
|
abs159270 |
Rabbit anti-CD81 Monoclonal Antibody (3D6) |
|
|
Exosomal auxiliary reagents |
abs9232 |
BCA Protein Quantification Kit |
|
abs9587 |
Exosome-specific lysate |
|
|
Exosome tracing |
abs42002237 |
DiI perchlorate |
|
abs45153674 |
DiO perchlorate |
|
|
abs45153692 |
DiR iodide |
|
|
abs47048165 |
DiD p-chlorobenzene sulfonate |
|
|
abs9819 |
PKH26 Red Fluorescent Cell Ligation Kit |
|
|
abs9820 |
PKH67 Green Fluorescent Cell Ligation Kit |
|
|
Exosome inhibitors |
abs810270 |
U-104 |
|
abs811902 |
GW 4869 |
|
|
abs813596 |
(R)-Lansoprazole |
|
|
abs814457 |
Neticonazole Hydrochloride |
|
|
abs814876 |
Lansoprazole |
|
|
abs818914 |
Heparin sodium salt |
|
|
Cell culture |
abs9430 |
Serum-free medium for exosomes |
|
abs993 |
Exosome-free fetal bovine serum |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. Email: worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
