- Cart 0
- English
Immune Cell Culture Strategy Expansion & Culture (4)
September 22, 2025
Clicks:128
overview
There are six basic processes of immune cell culture: sample preparation, cell sorting, typing and identification, expansion & culture, quality optimization and follow-up research. Xiao Ai has given you a detailed introduction to "Sample Preparation of Immune Cell Culture Strategy (1)" and "Cell Sorting of Immune Cell Culture Strategy (2)", and "Typing Identification of Immune Cell Culture Strategy (3)" Today we continue to learn the relevant knowledge of expansion & culture together.
From sample preparation to cell sorting and typing identification, after we finally obtained the target cells with high purity and good activity, we need to artificially simulate their growth environment in vivo in vitro, while ensuring sterility, suitable temperature and pH, and giving immune cells sufficient nutrition to make them grow and reproduce continuously. This process is expansion & culture, which can be mainly divided into culture system establishment, passage process, cryopreservation and resuscitation.
Expansion & Culture
1. Establishment of training system:
The cytokines and activation signals involved in different immune cells are quite different, so the culture system cannot be the same. The specific culture protocols of different sources of the same immune cell (such as PBMC source, umbilical cord blood source, iPSCs or ESCs) also differ. Xiao Ai listed some immune cell culture systems for reference. You can explore and optimize according to your own experimental needs to obtain the best culture scheme with high expansion efficiency, high cell purity and good activity.
(1) T cell (human) activation, proliferation culture and differentiation:
The activation of T cells involves two signals: the specific antigen stimulation signal produced by the binding of TCR/CD3 to the specific MHC-polypeptide complex on the surface of APC; The surface of APC produces non-specific costimulatory signals after acting on costimulatory molecules and corresponding receptors of T cells. The most commonly used for T cell activation in vitro are Anti-CD3 and Anti-CD28, which can fully activate T cells under the synergistic effect of the two [1]. In addition, we need to add different cytokines to induce T cells to differentiate in different directions (Figure 1).
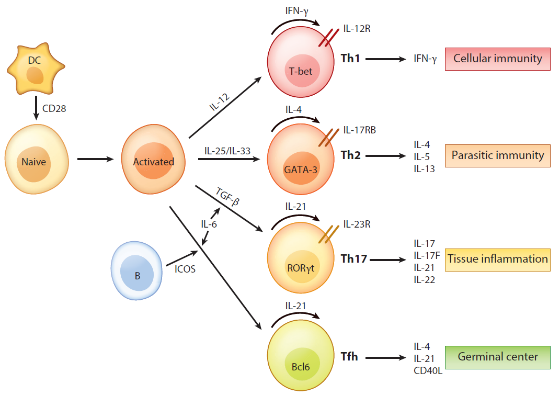
Figure 1 Regulation and function of cytokines in T cells [2]
1. Return the immune cell serum-free medium (abs9772) to room temperature, resuspend the isolated T cells, add CD3/CD28 magnetic beads (abs160019) (1: 1 with the number of cells) and 1% double antibody (abs9244) to promoteT cell activation and proliferation;
2. 300U/mL IL-2 (abs04045) was added to differentiate and proliferate into effector T cells; Or 5ng/mL IL-15 promotes T cell differentiation and proliferation into memory T cells [3].
The above is a T cell differentiation protocol in a literature. Xiaoai also summarized some cytokines that induce T cell differentiation in vitro (Figure 2) for your reference.
|
subtype |
cytokine |
medium |
culture time |
|
Th1 |
IL-2 + IL-12 |
RPMI1640+10% FBS+2μg/mL Anti-CD3+0.5 μg/mL Anti-CD28+1μg/mL Anti-IL-4+5ng/mL IL-2+10ng/mL IL-12+55μM β-mercaptoethanol |
96h |
|
Th2 |
IL2+IL4 |
RPMI1640 + 10% FBS + 2 μg/mL Anti-CD3 + 0.5 μg/mL Anti-CD28 + 1 μg/mL Anti-IFN-γ + 5 ng/mL IL-2 + 10 ng/mL IL-4 + 55 μM β-mercaptoethanol |
96h |
|
Th17 |
IL-6 + TGFβ |
RPMI1640 + 10% FBS + 2 μg/Ml Anti-CD3 + 0.5 μg/mL Anti-CD28 + 1 μg/mL Anti-IFN-γ + 1 μg/mL Anti-IL-2 + 1 μg/mL Anti-IL-4 + 20 ng/mL IL-6 + 1 ng/mL TGFβ1 + 55 μM β-mercaptoethanol |
96h |
|
Treg |
TGFβ1 |
RPMI1640+10% FBS+2μg/mL Anti-CD3+0.5 μg/mL Anti-CD28+1μg/mL Anti-IFN-γ+1μg/mL Anti-IL-4+1ng/mL TGFβ1+55μM β-mercaptoethanol |
96h |
Fig. 2 Protocol for inducing T cell differentiation in vitro [4]
Tips: Primary T cells are generally cultured in vitro for no more than three weeks, and the recommended culture time of T cells used in functional experiments is within 14 days.
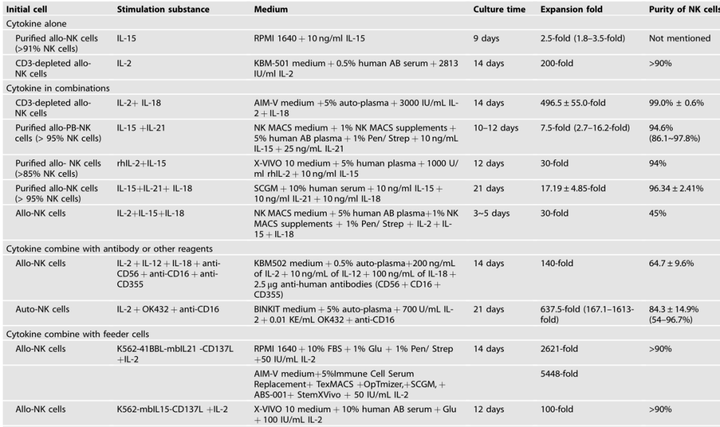
(2) In vitro expansion of PBMC (human)-derived NK cells [5]:
1. Adjust the density of isolated NK cells to 1 * 106 cells/mL, and resuspend them in serum-free medium containing immune cells (abs9772);
2. 200 U/mL IL-2, 10 ng/mL IL-15 and 1 * 106 cells/mL of K562 cells (inactivated by mitomycin treatment) were added. NK cells were passaged every 2-3 days during the 15-day expansion, supplemented with cytokines and freshly prepared K562 cells.
Of course, there are many methods for in vitro expansion of PBMC (human)-derived NK cells: cytokine − antibody fusions, feeder cells, or membrane particles. Xiaoai also found a review of 24.1 points, which listed in detail the culture medium, additives, culture days, expansion efficiency, and cell purity of different expansion schemes. You can save it as needed!
(3) Culture of monocytes (human) and induction of differentiation into macrophages [7]:
1. Add 1% double antibody (abs9244) and 10% fetal bovine serum superior grade (abs972) to immune cell serum-free medium (abs9772), use the above medium to resuspend the isolated monocytes with a cell density of 1x106 cells/mL, put them in an incubator at 37 ° C. and 5% CO2, and wait for about 1 hour to make the cells adhere to the wall;
2. Monocytes began to differentiate into generalized M0 macrophages after culture. On the third day after inoculation, fresh medium was replaced and 25 ng/mL granulocyte-macrophage colony stimulating factor (GM-CSF) was added to promote the M1 phenotype, or 50 ng/mL macrophage colony stimulating factor (M-CSF) to promote the M2 phenotype. On day 6, the fresh medium can be replaced again and 100 ng/mL LPS and 20 ng/mL IFN-γ are added to polarize the cells to the M1-macrophage phenotype, or 10 ng/mL M-CSF and 20 ng/mL IL-4 are added; The culture can be continued for 24 hours.
(4) Culture of dendritic cells [8]:
1. Dendritic cells generally do not proliferate when cultured in vitro, and can survive in the culture environment for 1 week or more. If cells need to be continuously cultured for more than one night,it is recommended to add 500 U/mL GM-CSF and 500 U/mL IL4 to immune cell serum-free medium (abs9772) to ensure the maintenance of the DC phenotype.
2. Dendritic cells at 10 ng/mL TNF-α, 5 ng/mL IL-1β, 15 ng/mL IL-6 and 10 ug/mL Prostaglandin E2 or 0.2 U/mL Heparin and 50 μg/mL Keyhole Limpet Hemocyanin. Or 0.2 U/mL Heparin.
2. Passage process
Immune cells with good adherence:
Discard the 1-day medium in the culture flask → wash the cells 1-2 times with sterile PBS → add an appropriate amount of trypsin-EDTA solution to digest the cells → add an appropriate amount of complete medium containing serum after the cells are shed → blow the cells with a pipette and transfer them to a centrifuge tube for 200g, 5min → discard the supernatant, add an appropriate amount of complete medium to resuspend the cells for counting → dilute the cell suspension to the appropriate seeding density and transfer them to a new culture flask for culture
Semi-adherent and suspended cells:
Pipette fully pipetting the cells so that the cells are evenly dispersed in the culture medium → Cell counting Resuspend the cells to the appropriate seeding density with fresh culture medium → Dispense into new culture flasks and put in an incubator for culture
3. Cryopreservation and resuscitation [9]:
(1) Cryopreservation: Cryopreservation can be performed when the cell density reaches 107cells/mL, and the step-by-step freezing method is adopted. The specific steps are as follows:
1. The suspended cells were centrifuged at 500g at room temperature for 10min to collect cell pellets, and the adherent cells were digested with trypsin and terminated, and then centrifuged to collect;
2. Discard the supernatant, resuspend the cell pellet in the cryopreservation solution, subpack it in 1mL and label it;
3. Place the cryopreservation tube at-20 °C for 1 h; -80 °C overnight; Transfer to liquid nitrogen for long-term storage.
(2) Recovery:
1. Take out the cells from liquid nitrogen and quickly thaw the cells in a 37 °C water bath;
2. Transfer the cells to a 15 mL centrifuge tube containing 12 mL of medium;
3. 500 g centrifuge at room temperature for 5 minutes and discard the supernatant;
4. Add culture medium to resuspend cells for subsequent culture.Tips: Resuscitated immune cells It is recommended to count the number of cells in the cell suspension by blood cell count and determine the cell viability by trypan blue dye.
That's all for today's explanation, the next quality optimization!
References:
[1] Gunnlaugsdottir, B., Anti-CD28-induced co-stimulation and TCR avidity regulates the differential effect of TGF-1 on CD4 + and CD8 + naive human T-cells. International Immunology, 2004. 17 (1): p. 35-44.
[2] Dong, C., Cytokine Regulation and Function in T Cells. Annual Review of Immunology, 2021. 39 (1): pp. 51-76.
[3] Nguyen, D.N., et al., Polymer-stabilized Cas9 nanoparticles and modified repair templates increase genome editing efficiency. Nature Biotechnology, 2019. 38 (1): pp. 44-49.
[4] Sekiya, T. and A. Yoshimura, In Vitro Th Differentiation Protocol, in TGF-β Signaling. 2016. p. 183-191.
[5] Li, F., et al., CCL5-armed oncolytic virus augments CCR5-engineered NK cell infiltration and antitumor efficiency. Journal for Immunotherapy of Cancer, 2020. 8 (1).
[6] Fang, F., et al, Advances in NK cell production. Cellular & Molecular Immunology, 2022. 19 (4): p. 460-481.
[7] K.Plaisance-Bonstaff, C.F., D. Wyczechowska, et al., Isolation, Transfection, and Culture of Primary Human Monocytes. JVis Exp, 2019 (154): p. 10.3791/59967.[8] Boudewijns, S., et al., Autologous monocyte-derived DC vaccination combined with cisplatin in stage III and IV melanoma patients: a prospective, randomized phase 2 trial. Cancer Immunology, Immunotherapy, 2020. 69 (3): p. 477-488.
[9] Raulf, M., T Cell: Primary Culture from Peripheral Blood, in Allergy. 2019. p. 17-31.
Recommended by Xiaoai in this issue
|
Item number |
Product name |
Specifications |
|
abs9772 |
Serum-free medium for immune cells |
1L |
|
abs04045 |
Recombinant Human IL-2 Protein |
10ug/50ug/100ug |
|
abs160019 |
CD3/CD28 Magnetic Bead |
1.5 mL × 4/1.5 mL × 8 |
|
abs972 |
Fetal bovine serum (superior grade) |
500mL |
|
abs9244 |
Penicillin-streptomycin solution (100 ×, double antibody) |
100mL |
|
abs04123 |
Recombinant Human IFN-γ Protein |
10ug/50ug/100ug/500ug/1mg |
|
abs04698 |
Recombinant Human IL-4 Protein |
10ug/100ug/500ug |
|
abs04079 |
Recombinant Human IL-13 Protein (C-6His) |
10ug/50ug/500ug/1mg |
|
abs7011 |
25 cm ² cell culture flask (50 mL, air permeable cap) |
200 pcs/box |
|
abs7033 |
Cell culture plate (standard clear 6-well plate) |
50 pcs/box |
|
abs7054 |
25mL disposable serum pipette |
200 pcs/box |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. Email: worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
