- Cart 0
- English
ADC drug development overall solution
August 26, 2025
Clicks:91
ADC combines the precise navigation of antibodies with the powerful killing of toxins,which is a revolutionary solution for targeted cancer therapy. Absin can provide researchers with ADC antibody internalization detection reagents, ADC cytotoxins, ADC Linker, ADC Linker with Payload, ADC site-directed coupling kits, ADC popular target proteins, ADC positive reference antibodies, ADC pathological antibodies (Start), ADC overexpression drug target cell lines and other reagents and related technical services to help accelerate the development of ADC drugs.
Introduction to ADC drugs
Antibody-Drug Conjugate (ADC) is a combination of highly selective monoclonal antibodies (mAb) and highly cytotoxic small molecule chemical drugs, namely payloads (Payloads), through a Linker (Linker) The drug obtained by conjugation (Figure 1). ADC combines the targeting of monoclonal antibodies to tumor cells and the powerful tumor lethality of cytotoxic drugs, and overcomes the problems of weak cytotoxicity of monoclonal antibodies and high systemic toxicity of cytotoxic drugs, and has the therapeutic advantage of 1 +1 > 2.

The mechanism of action of ADCs is complex and usually requires drug internalization followed by intracellular processing and payload release. A typical model assumption for the action of ADC is as follows: the antibody binds to the target antigen, then internalizes, and is transported into the lysosome through the endosome. In the lysosome, the linker or antibody part of the ADC degrades and releases the payload, which further acts to produce cytotoxicity and thus kills tumor cells. But the actual situation is more complicated, and there are clear differences between ADCs (Figure 2).
ADC Drug Design Elements
1. Target Antigen (Target Antigen)
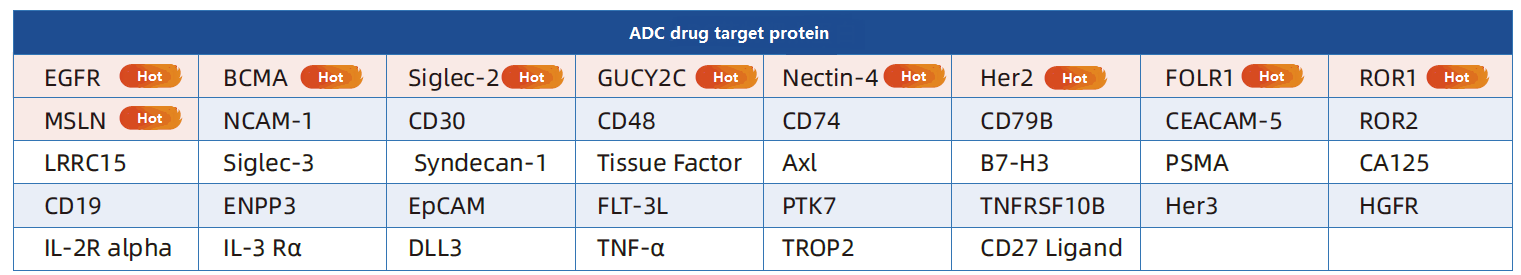
ADC drug design starts with the target antigen, and its selection needs to meet: (1) Specificity, high expression of tumor cells, low or no expression of normal cells; (2) It is a tumor cell surface antigen; (3) It can efficiently induce the internalization process, etc.
The selection of target protein is directly related to the targeting, therapeutic effect and safety of ADC. Aibxin is deeply involved in the field of target protein research and development, and has a resource library of more than 2,800 high-quality target proteins. Among them, ADC popular target proteins are fully covered, and they are widely used in immunogen preparation, high-throughput antibody screening, cell signaling pathway research and functional mechanism Verification and other scenarios.
The efficacy of most ADC drugs is mainly based on the drug release after internalization, that is, the ADC-antigen complex formed by the binding of antibodies and tumor cell surface antigens needs to be effectively induced to internalize, enter tumor cells, and be transported and degraded intracellularly to achieve small molecular drug release. The internalization of ADC was visualized by cellular immunofluorescence (Figure 3), and the internalization efficiency was quantified by DT3C content detection (Figure 4). DT3C (Diphtheria Toxin Fragment A and Streptococcus Protein G C1, C2, C3) It is a recombinantly expressed fusion protein that has high affinity with the Fc terminus of the antibody, and the DT3C molecule bound to the antibody enters the cell together when the antibody is endocytosed. DT3C can bind to any lgG-type antibody and thus can be used to detect the internalization efficiency of antibodies from different species.

2. Antibody (Antibody)
Antibodies in ADC drugs need to meet: high specificity, strong target binding ability, low immunogenicity, and low cross-reactivity, so as to achieve more efficient uptake of ADC drugs by tumor cells and longer half-life of ADC drugs in serum.
Immunoglobulin G (IgG) is the primary antibody backbone used in ADCs. Therefore, ADC drugs in clinical and preclinical studies usually choose IgG as the antibody targeting the antigen of interest. IgGs can be divided into four subtypes: IgG1, IgG2, IgG3, and IgG4. Among them, IgG1 is the most studied and used ADC antibody because it can better balance the relationship between long blood half-life and strong immune activation, and has high natural abundance. IgG4 is also often used in some ADC drug designs that require higher immunogenic response due to its low immune activation effect.
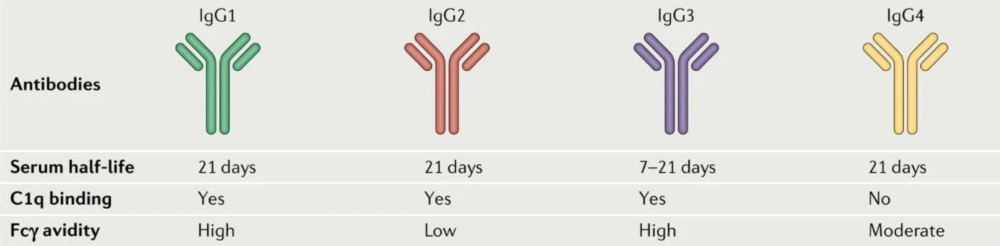
Figure 5 Comparison of different IgG [2]
3. Payload
The cytotoxic load drug carried by ADC, that is, payload, is its most important effector component. At present, commonly used cytotoxic molecules include microtubulogenesis inhibitors, DNA damage factors and DNA transcription inhibitors.
When choosing small molecule drugs, you need to meet:
(1) IC50 values are as low as nanomolar or even picomolar levels;
(2) It is not easy to cause ADC drugs to accumulate after coupling with antibodies to ensure a long circulation time in the body;
(3) The ADC drugs themselves and after formation need to have low immunogenicity;
(4) It is stable enough in aqueous solution (blood) and has a suitable reaction site to be coupled to the antibody through a linker, and its biological activity can still be guaranteed after coupling;
(5) It can be synthesized by a relatively economically efficient process.
4. Linker
Linker plays a vital role in ADC results, which will affect the pharmacokinetic parameters, therapeutic index and efficacy of ADC. Linker maintains the stability of the ADC complex in the bloodstream and minimizes non-targeted effects. Moreover, Linker should be able to rapidly release cytotoxic drugs during the internalization of ADCs by tumor cells.
According to the cleavable properties, Linkers are divided into cleavable Linkers and non-cleavable Linkers, and cleavable Linkers can be further divided into three types: hydrazone, disulfide and peptide Linkers (Table 1). Non-cleavable Linkers include non-degradable thioethers or Maleimidocaproyl (MC), which are degraded by lysosomal enzymes after Payload internalization into target cancer cells in the ADC. Reasonable selection and optimization of Linker and its coupling strategy with Payload can effectively improve the therapeutic effect of ADC and reduce off-target effects.
|
Type |
trait |
|
Hydrazone |
The acid-sensitive hydrazone Linker is susceptible to acid hydrolysis at low pH (4-6) of cancer cell endosomes and lysosomes, which facilitates the efficient release of Payload. |
|
Disulfide |
Disulfide Linkers have good stability in blood circulation, and they use higher concentrations of glutathione in cancer cells for release. Cancer cells are rich in glutathione, which is related to the high stress state caused by tumor cell growth and hypoxia. |
|
Peptide |
Lysosomal protease-sensitive peptides such as: cathepsin B can act on tumor-specific proteases that cleave the dipeptide bond of ADC within tumor cells; Valine citrulline is a cathepsin B-sensitive dipeptide; β-glucuronidase is an enzyme overexpressed in many tumors. β-glucuronide is sensitive to this enzyme and can be degraded and hydrolyzed by it. Therefore, β-glucuronide can be used as a protease-sensitive Linker for selective release of Payload. |
5. Coupling technology
Coupling technology connects antibodies to payloads and is closely related to the final Drug to Antibody Ratio (DAR). DAR is the average number of payloads linked to each mAb, which can be measured by HPLC-MS, etc. It has a significant impact on drug pharmacology and activity, and is a key parameter in the later stage of ADC development. Because the number of ADCs taken by tumor cells is limited, higher DAR is conducive to improving efficacy, but small molecule drugs are highly hydrophobic. Too high DAR will cause ADCs to aggregate, shorten the circulating half-life in vivo and increase toxic and side effects. Therefore, preclinical and clinical DAR is usually between 2-8.
Coupling methods are mainly divided into non-site-directed coupling and site-directed coupling. The non-site-directed coupling method was used in the early days, mainly by lysine or cysteine coupling. Site-directed coupling means specific coupling through genetically engineered sites to achieve a more uniform ADC, which can achieve the connection of cytotoxins at specific sites.
Enzymatic coupling method
Sortase A (abs05842) cleaves between threonine (T) and glycine (G) residues by recognizing a specific peptide sequence on the target protein, typically LPXTG (X can be any amino acid) (Fig. 6). Sortase A-mediated antibody coupling (SMAC) technology allows efficient coupling of toxins to antibodies at preset sites on the antibody, resulting in a highly stable coupling product with uniform DAR values.
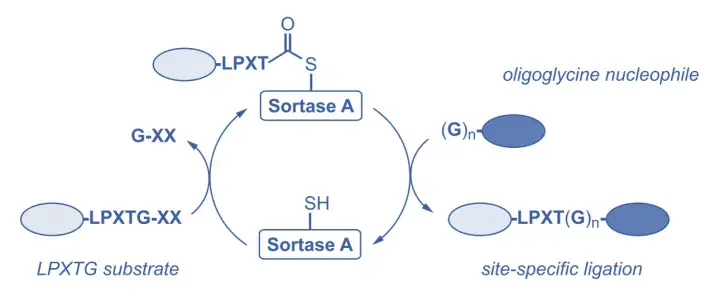
Figure 6 Transpeptidase/Sortase (Sortase) and transpeptidase reaction
Coupling strategy based on azodextran engineering technology
N-Glycan engineering techniques based on β-1, 4-galactosyltransferase (GalT) and α-2, 6-sialyltransferase (SialT) were used for coupling. In vitro, terminal sialic acids can be introduced using an enzymatic reaction of galactosyl and sialyltransferase. These sialic acids can be oxidized by periodate to yield aldehyde groups and are further covalently coupled to Payload via oxime linkages. The main advantage of this technique is that this strategy is highly reproducible regardless of the heterogeneity of N-Glycan, so it can be used for coupling any mAb containing various N-Glycan to Payload.
Aibixin ADC toxin antibody site-directed coupling kit (abs580253) is easy to operate, does not require complicated operations such as antibody engineering, and can quickly realize site-directed coupling of monoclonal antibodies. The coupling product is homogeneous and stable. It is suitable for site-directed coupling in the coupling stage and early coupling research of ADC.
Coupling principle:
(1)Azide modification of antibody
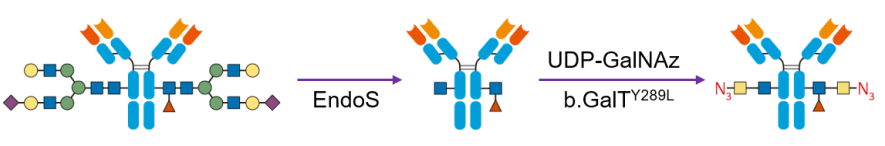
Acetylglucosamine on the conserved N sugar chain of the monoclonal antibody constant region was first exposed using glycosidase (EndoS) (blue squares), and then acetylgalactosamine with azide functionality (yellow squares-N3) was attached to acetylglucosamine using a bovine galactosyltransferase mutant (b. GalTY298L).
(2)Toxin molecule linkage

Biotin, fluorescein, or toxin molecules (e.g. endo-BCN-PEG4-Val-Cit-PAB-MMAE, abs823512) can then be attached to the monoclonal antibody using a copper-free catalyzed alkyne azide cycloaddition reaction (e.g. SPAAC).
Validation data
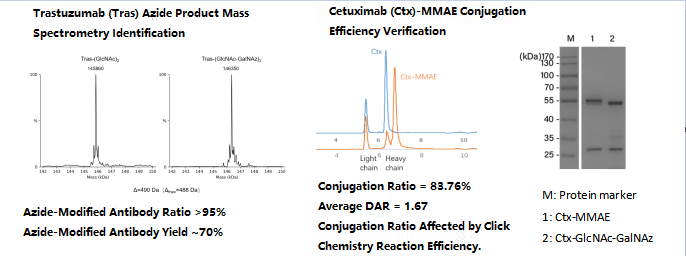
ADC process development, CMC and preclinical studies
ADC drug production covers three modules: antibodies, cytotoxic drugs/linkers, ADC APIs and preparations. Each module requires process development and verification, and quality control requirements for engineered cells, starting materials and reagents must be formulated. The intermediate mass control for coupling production is flexible, which tests the research and development capabilities of the process and quality control system. The difference in the properties of antibodies and cytotoxins makes production challenging, and the process characterization study is of great significance to subsequent batches. The CMC study has 4 parts: 1. Antibodies; 2. a load-linker intermediate; 3. ADC APIs; 4. Formulation part. At the same time, special quality control indexes such as drug-antibody ratio, ligation site, and drug load distribution should be introduced, and free load and antibody should be controlled to study their effects on binding efficacy and plasma stability.
Unique challenges are presented for pharmacokinetic (PK) and pharmacodynamic (PD) characterization due to the complexity and diversity of ADCs, as well as the low levels of cytotoxic drugs released in biological samples. In addition, the selection of bioanalytical methods and the accuracy of testing are also considerations in safety evaluation.
In pharmacokinetic (PK) and pharmacodynamic (PD) studies, we provide comprehensive experimental reagents and technical service support (Luminex detection services, high-sensitivity electrochemiluminescence (MSD), DSP spatial multi-omics), products and services cover the entire process from basic cell experiments to complex tissue analysis, providing one-stop solutions for pharmacological and toxicological research.
|
General Cytotoxicity |
Mitochondrial Toxicity |
Histopathological Detection |
|
Cell Viability Assay: 2D Luminescent Cell Viability Assay (abs50065) |
Mitochondrial Membrane Potential Assay: Mitochondrial Membrane Potential Assay Kit (JC-10) (abs50017)
|
Multiplex Fluorescent Immunohistochemistry (IHC/IF): 4-Color Multiplex Fluorescent IHC Staining Kit (Anti-Mouse/Rabbit Universal Secondary Antibody) (abs50012)
|
|
Cell Apoptosis Assay: Annexin V-FITC/PI Apoptosis Detection Kit (abs50001)
|
Reactive Oxygen Species (ROS) Assay: Reactive Oxygen Species ROS Assay Kit (Green Fluorescence) (abs580232)
|
|
|
Cytotoxicity Assay: MTT Cell Proliferation and Cytotoxicity Assay Kit (abs50010)
|
Glucose/Galactose Assay: Glucose Microplate Assay Kit (abs580025)
|
Conventional IHC/IF Reagents: Ready-To-Use High-Efficiency IHC Secondary Antibody Kit (abs957)
|
References
[1] Kyoji Tsuchikama, et al. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell. 2018Jan;9(1):33-46[2] Joshua Z Drago, et al. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021 Jun;18(6):327-344.
ADC antibody internalization assay
|
Item No.-Specification
|
Name
|
|
Recombinant Aureus Sortase A Protein
|
|
|
Lysosomal Red Fluorescent Probe (FluoLyso Red)
|
|
|
Lysosomal Deep Red Fluorescent Probe (FluoLyso Deep Red)
|
|
|
Rapid FITC Antibody (Protein) Labeling Kit
|
|
ADC cytotoxins
|
Item No.-Specification |
Name |
|
Daun02 |
|
|
Aldoxorubicin |
|
|
Campathecin |
|
|
MMAF-OMe |
|
|
Vipivotide tetraxetan |
|
|
McMMAF |
ADC Linker
|
Item No.-Specification |
Name |
|
Fmoc-Val-Cit-PAB-PNP |
|
|
Val-cit-PAB-OH |
|
|
Fmoc-Val-Cit-PAB |
|
|
MC-Val-Cit-PAB |
|
|
Indole-C2-amide-C2-NH2 |
|
|
DBCO-acid |
ADC site-directed coupling kit
|
Item No.-Specification |
Name |
|
ADC toxin antibody site-directed coupling kit |
|
|
ADC toxin antibody site-directed coupling kit |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. Email: worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
