- Cart 0
- English
Animal Modeling Special Topic: Is EAE modeling asymptomatic? These details you must know
August 22, 2025
Clicks:115
Animal models are life carriers carefully constructed by medical researchers to simulate human disease manifestations. They accurately reproduce the complex process and key characteristics of diseases. It is mainly used in experimental physiology, experimental pathology and experimental therapeutics (including new drug screening) research. For example, some classic animal models-EAE model, diabetes model, enteritis model, Parkinson's model, AD model, etc.
experimental autoimmune encephalomyelitis (EAE) is the most common animal model of MS and has many clinical and pathophysiological features. EAE models are diverse and reflect diverse clinical, immunological, and histological features of human MS. The mouse model of EAE by active induction is the most easily inducible model with robust, replicable results. It is particularly suitable for studying the effects of drugs or specific genes on autoimmune neuroinflammation using transgenic mice. Therefore, mice were immunized by subcutaneous injection of central nervous system homogenate or myelin protein peptides.
Due to the low immunogenicity of these peptides, potent adjuvants were used. The susceptibility and phenotype of EAE depend on the selected antigen and rodent strain. C57BL/6 mice are the most commonly used strain in transgenic mouse construction and are responsive to myelin oligodendrocyte glycoprotein (MOG). The immunogenic epitope MOG35-55 was suspended in complete Freund's adjuvant (CFA) for immunization, and pertussis toxin was injected on the day of immunization and two days later. Mice develop "classical" self-limiting monophasic EAE within 9-14 days post-immunization, manifesting as ascending flaccid paralysis. Mice were evaluated daily for 25-50 days using a clinical scoring system.
The success rate of EAE model induction is influenced by several factors, including the species and sex of mice, the dose of pertussis toxin, the use of adjuvants, the degree of emulsification, and the extensive experimental foundation and modeling experience. Today, Xiaoai focuses on explaining the details that need attention in the process of EAE modeling.
EAE modeling method
Experimental animals: 6-8 weeks old female C57BL/6 mice
Animal adaptation: Adapt the animals to the experimental environment for 1 week, keep the temperature, humidity and light conditions stable, and enable them to freely obtain food and water.
3. EAE model establishment
(1) MOG (35-55) solution preparation: MOG (35-55) (abs815889) polypeptide powder is fully dissolved in pre-cooled (4 ℃) sterile PBS, and the final concentration is 2.0 mg/mL.
Note: Polypeptides are changeable at room temperature after dissolution, are easy to fail after long-term storage, and there is a risk of freeze-thaw stability. It is recommended to be prepared and used immediately.
(2) Preparation of Freund's complete adjuvant (CFA) solution: Suspend heat-inactivated Mycobacterium tuberculosis H37 Ra in CFA (abs9270) or Freund's incomplete adjuvant (abs9271), and through thorough stirring, heat-inactivated Mycobacterium tuberculosis The final concentration reaches 8.0 mg/mL.
WARNING: Heat-inactivated Mycobacterium tuberculosis stimulates innate immune responses, avoid inhalation, ingestion, and contact with skin and eyes. Needle stick wounds are strictly avoided when preparing the adjuvant, as it may cause granuloma or induce autoimmune reactions.
※ Effects and concentrations of heat-inactivated Mycobacterium tuberculosis
① Enhance immunogenicity
Provide a "danger signal": the cell wall components of Mycobacterium tuberculosis (such as mycobacterial acid, lipoarabinomannan, etc.) can activate the innate immune system through Toll-like receptors (TLR2/4) and promote the maturation of antigen-presenting cells (APC) and the release of inflammatory factors (such as IL-12, TNF-α), thereby enhancing the immune response to MOG35-55.
Induction of Th1/Th17 polarization: Mycobacterium tuberculosis components can drive the differentiation of CD4 + T cells into pro-inflammatory Th1 (IFN-γ-secreting) and Th17 (IL-17-secreting) subsets, which are the core mechanisms in the pathogenesis of EAE.
② Formation of granuloma reaction
Continuous stimulation of Mycobacterium tuberculosis can lead to the formation of granulomas at the injection site, prolong antigen release time and maintain a chronic inflammatory state.
③ Final concentration range: 4-10 mg/mL. Too high concentration (> 10 mg/mL) may lead to excessive inflammation or animal death.
④ Absin's CFA (abs9270) contains 1mg of heat-inactivated dried Mycobacterium tuberculosis (H37Ra, ATCC 25177) per ml.
(3) Preparation of antigen emulsion: MOG (35-55) polypeptide solution and CFA solution were added to two 10mL glass syringes according to a volume ratio of 1: 1, and the two syringes were connected through a three-way. The antigen was emulsified to a water-in-oil state by repeated bolus injection on ice for 40 min-2 h, and the concentration of MOG (35-55) polypeptide reached 1.0 mg/mL.
Note: ① The purity of the peptide must be > 98%;
② In the process of making emulsion, the loss is very large (generally 1/3-1/2 loss). Theoretically, one mouse needs to be injected with 200ug of MOG peptide, and ten mice need to be injected with 2mg, but with the loss amount, immunized ten mice need to weigh 4mg to prevent insufficient after the loss. If the lotion is left, it can be put in a 4-degree refrigerator and used within a week without any problem;
③ Plastic syringes cannot be used for emulsion preparation to avoid interference of organic solvents and protein adsorption;
④ The emulsion can be stored for several days before immunization. Wait at least 30 minutes after preparing the emulsion to see if it is stable, the solution should be white, viscous and without phase separation. Prior to immunization, the solution was drawn into one of the two syringes and the needle was attached.
(4) Preparation of PTX solution: 50 μg of pertussis toxin (abs42024900) was reconstituted in 500 μL of ddH2O (double distilled water) to obtain 100 μg/mL stock solution, stored at 4 °C. The PTX stock solution was diluted in sterile PBS (abs962) to achieve a final concentration of 3.0 ng/μL;
Warning: Whooping cough toxin has a variety of biological effects. Avoid inhalation, ingestion, and contact with skin and eyes.
(5) Anesthetized mice: anesthetized mice with isoflurane;
(6) EAE model: First, the anesthetized mice were shaved, and 50.0 μL of MOG (35-55) polypeptide solution emulsified by CFA was subcutaneously injected subcutaneously in the back, armpit and groin (ensuring that a globular mass is formed subcutaneously, which should persist throughout the experiment). On the day and 48 h after MOG (35-55) polypeptide emulsion injection, PTX was injected intraperitoneally at 300 ng/animal to enhance disease induction (color marking was performed at the base of the mouse tail to ensure that individual mice could be easily identified for daily evaluation).
EAE model monitoring
(1) Clinical score and weight monitoring
Body weight and clinical score should be assessed daily. The onset of EAE is usually associated with weight loss, and the clinical manifestations and weight changes of EAE mice are monitored and recorded daily.
① Standardized scoring system 0-5 subscale (most commonly used): starting from day 0, continuous observation for 30 days or more. Scoring criteria: 0 points: asymptomatic. 1 point: tail weakness or paralysis. 2 points: hind limb weakness. 3 points: hind limb paralysis. 4 points: quadriplegia. 5 points: Death.
② Weight monitoring: EAE mice are usually accompanied by weight loss (> 10% indicates the success of the model), which needs to be recorded daily. Continuous weight loss may require ethical intervention.
Note: When mice develop clinical signs of EAE, it is important to ensure that the water bottle is still accessible and to keep the food on the cage floor.

Figure 1 Picture of immunized mice with EAE symptoms

Figure 2 Typical disease progression (n = 6)
(2) Behavioral testing
① Motor function assessment
Rotarod experiment (Rotarod): Acceleration mode (4-40 rpm, completed within 5 minutes) records the drop time, reflecting coordination and endurance.
Gait analysis (CatWalk or footprint method): quantify stride length, base width, swing speed.
② Depression-like behavior detection
Forced Swimming Experiment (FST): Time of immobility was recorded (reflecting desperate behavior).
Tail suspension test (TST): an auxiliary verification of depressive phenotype.
(3) Histopathological verification
① Material collection and processing
Perfusion fixation: After 4% paraformaldehyde perfusion, spinal cord (lumbar expansion segment) and brain tissue were taken.
Section preparation: paraffin section (5 μm) or frozen section (10 μm).
② Dyeing method
|
Type of staining |
Detection target |
Interpretation of results |
|
HE staining |
Inflammatory cell infiltration |
Perivascular "cuff-like" infiltration (score 0-3) |
|
LFB staining |
Demyelination |
Percent blue-stained regions missing (ImageJ quantification) |
|
Immunohistochemistry |
Microglia (Iba1), astrocytes (GFAP) |
Positive cell density/activated morphology |
(4) Molecular biology verification
① Flow cytometry
Cell subset analysis: Th1 (CD4 + IFN-γ +), Th17 (CD4 + IL-17 +), Treg (CD4 + FoxP3 +).
Sample source: spleen, lymph nodes, central nervous system single cell suspension.
② Cytokine detection
ELISA/liquid phase chip: pro-inflammatory factors: TNF-α, IL-6, IL-17
Anti-inflammatory factors: IL-10, TGF-β
qPCR: Cytokine mRNA expression in spinal/brain tissue.
③ Protein expression analysis
Western Blot:
Inflammatory pathway proteins: NF-κB p65, pSTAT3
Neuroprotection markers: BDNF, NGF
Model success criteria
Basic criteria: incidence > 80% (clinical score ≥ 2); Pathologically confirmed HE showed inflammatory infiltration + LFB confirmed demyelination.
Advanced criteria: behavioral abnormalities (Rotarod time ↓ 50%); Molecular marker: Th17 ratio ↑ 2-fold, and IL-17 levels in the CNS were significantly increased.
Analysis of asymptomatic causes of EAE modeling
|
Frequently asked questions |
Possible causes |
Solution |
|
Induration at injection site |
Insufficient emulsification of adjuvant: Incomplete emulsification of CFA (Freund's complete adjuvant) and antigen (such as MOG35-55) may lead to oil-water separation, and the mineral oil in the adjuvant cannot be metabolized, resulting in induration. |
Verify the emulsification effect by visual inspection: drop the emulsified mixture into water, and if it keeps the bead shape and does not scatter, it means that the emulsification is sufficient; If it disperses quickly, it needs to be re-emulsified. |
|
Too shallow injection site or technical problems: If injected under the skin rather than at the subcutaneous junction with the muscle (recommended site), the adjuvant may be encapsulated by fibrous tissue and delayed release. |
Adjust the injection method: Choose multi-point injection subcutaneously in the back (such as interscapular area) or subcutaneously in the groin to avoid single point accumulation. The total injection volume per mouse is recommended to be controlled at 100-200 μL (injected at 2-4 points) to reduce local pressure. |
|
|
No evident EAE symptoms |
Emulsion not absorbed, CFA/PTX failed |
Asymptomatic patients can be tested by flow cytometry (detecting the ratio of Th1/Th17 cells in spleen/lymph nodes), ELISA (detecting cytokines such as IFN-γ and IL-17 in serum), and histopathology (taking spinal cord sections to observe inflammatory infiltration and demyelination) to verify whether immunity is activated. |
Featured application cases of EAE animal models
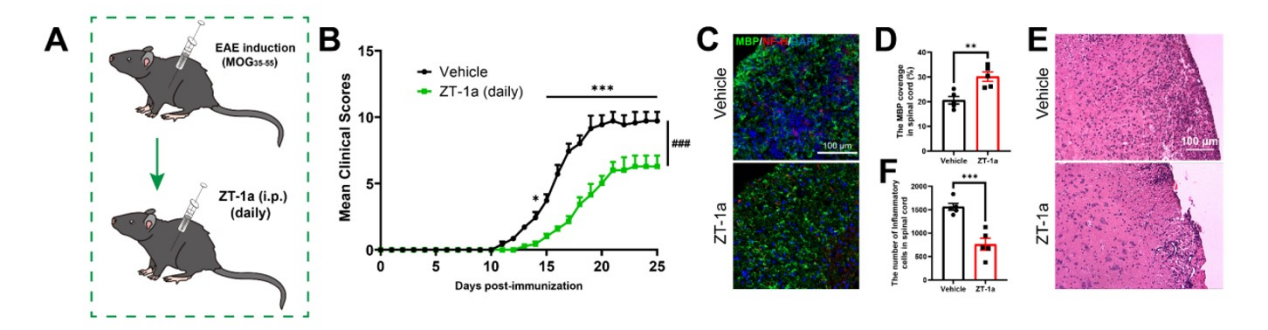
1. PTX constructed a C57BL/6 mouse EAE model, successfully revealed the core role of SPAK signaling in driving MS pathology by regulating the choroid plexus barrier, and provided preclinical evidence for SPAK-NKCC1 inhibitors (such as ZT-1a and bumetanide) in the treatment of MS.
Cited literature : J Neuroinflammation. 2025 Mar 13; 22: 80. IF: 9.3
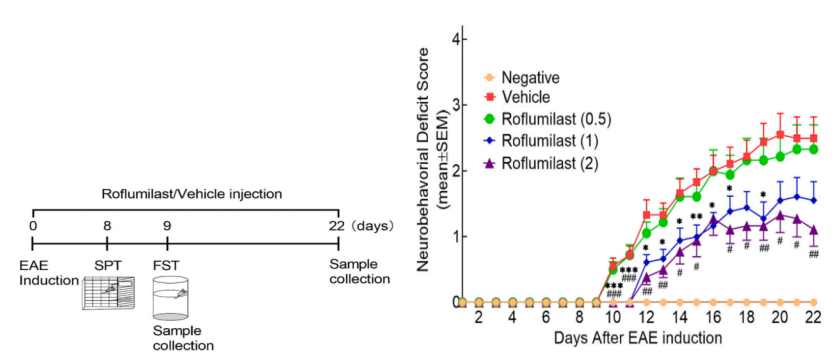
2. The EAE model of SD rats was constructed by PTX to study the role of Roflumilast in regulating neuroinflammation, improving motor function and depressive symptoms in multiple sclerosis (MS).
Cited literature : J Affect Disord. 2024 Apr 1: 350: 761-773. IF: 6.6
References:
[1] Qi C, Wang Y, Li X, et al. Target inhibition of SPAK in choroid plexus attenuates T cell infiltration and demyelination in experimental autoimmune encephalomyelitis. J Neuroinflammation. 2025;22(1):80.
[2] Wang Z, Zhang Y, Chai J, et al. Roflumilast: Modulating neuroinflammation and improving motor function and depressive symptoms in multiple sclerosis. J Affect Disord. 2024;350:761-773.
Recommended EAE Modeling Reagents
|
Item number |
Product name |
Specifications |
|
abs42024900 |
Whooping cough toxin |
50μg |
|
abs815889 |
MOG(35-55) |
1mg |
|
abs9270 |
Freund's complete adjuvant |
10mL |
More modeling reagent recommendations
|
Item number |
Product name |
Disease model |
|
abs050289 |
Recombinant Human MOG His Tag Protein(His Tag) |
EAE model |
|
abs05520 |
Recombinant Human MOG Protein(His Tag) |
EAE model |
|
abs05465 |
Recombinant Mouse MOG Protein(His Tag) |
EAE model |
|
abs45128173 |
β-Amyloid Polypeptides (1-42) |
AD model |
|
abs47014848 |
Lipopolysaccharide (O55: B5) |
Inflammatory model |
|
abs42020800 |
Lipopolysaccharide (O111: B4) |
Inflammatory model |
|
abs47014899 |
Human very low density lipoprotein |
Atherosclerosis |
|
abs47014903 |
Human oxidized low density lipoprotein |
Atherosclerosis |
|
abs47014905 |
Human Red Fluorescent Labeled Oxidized Low Density Lipoprotein |
Atherosclerosis |
|
abs816876 |
Cholesterol |
Atherosclerosis |
|
abs06612 |
Decorin |
Fibrosis/Tumor/Bone and Joint Disease Model |
|
abs42156029 |
Persartane |
Arthritis/lupus nephritis |
|
abs47006104 |
Collagen Type II (Chicken) |
Rheumatoid arthritis |
|
abs81648 |
Urethane |
Lung tumor model |
|
abs817895 |
Bleomycin sulfate |
Pulmonary fibrosis model |
|
abs812855 |
Vitamin A acid |
Osteoporosis |
|
abs9206 |
Egg white albumin (refined grade) |
Asthma model |
|
abs812888 |
Streptozocin |
Diabetic model |
|
abs816779 |
D-galactose |
Acute aging model |
|
abs9107 |
Phorbol ester |
Skin tumor model |
|
abs814897 |
MPTP HCl |
Parkinson's syndrome model |
|
abs42070403 |
6-hydroxydopamine hydrobromide |
Parkinson's syndrome model |
|
abs42147674 |
Sodium deoxycholate |
Chronic atrophic gastritis |
|
abs817976 |
Indomethacin |
Chronic atrophic gastritis |
|
abs47050756 |
Aspirin |
Acute gastritis |
|
abs810777 |
(+)MK-801 maleate |
Schizophrenic model |
|
abs812832 |
(+)-Bicuculline |
Convulsion model |
|
abs9192 |
Dextran sulfate sodium salt (molecular weight 36,000-50,000) |
Colitis model |
|
abs811942 |
L-butylthionine-sulfoxide imine (L-BSO) |
Anxiety model |
|
abs45129586 |
Cerulin |
Acute pancreatitis |
|
abs47033242 |
L-arginine |
Acute pancreatitis |
|
abs810716 |
Doxorubicin hydrochloride ( Doxorubicin) |
Cardiac disease model |
|
abs810466 |
Cisplatin (Cisplatin) |
Acute kidney injury model |
|
abs42025971 |
Aminonucleoside puromycin |
Nephropathy model |
|
abs45150353 |
Human angiotensin II |
Cardiovascular model |
|
abs42016150 |
D-(+)-galactosamine hydrochloride |
Animal model of liver cirrhosis |
|
abs813707 |
Lithocholic acid |
Cholecystitis model |
|
abs813570 |
Cysteamine hydrochloride |
Duodenal ulcer |
|
abs813590 |
Desoxycorticosterone acetate |
Hypertension |
|
abs47000420 |
Scopolamine |
Epilepsy model |
|
abs47001830 |
Pilocarpine |
Epilepsy model |
|
abs9271 |
Freund's incomplete adjuvant |
Immunoadjuvant |
|
abs45126715 |
Muramyl dipeptide |
Immunoadjuvant |
|
abs9933 |
ODN 1018 sodium |
Immunoadjuvant |
|
abs9983 |
ODN 2395 sodium |
Immunoadjuvant |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. Email: worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
