- Cart 0
- English
CoIP Experiment Frequently Asked Questions
August 05, 2025
Clicks:1265
Immunoprecipitation (IP/CoIP) is based on the specific affinity of antibodies and proteins. By capturing antibodies that specifically bind to proteins or antigens, the target proteins are captured and enriched from complex samples, and proteins or other biological macromolecules interacting with them can be measured.
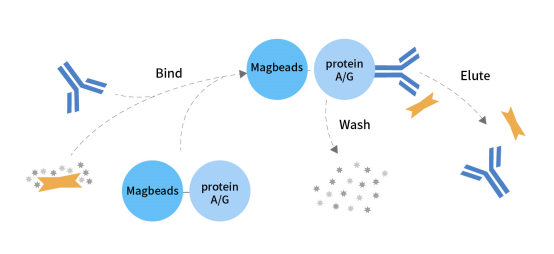
CoIP Schematic
Setting Method of CoIP Experimental Control
There are three common ways:
1) The first method is that the experimental group is transfected with the decoy protein and the tag protein, while the control group is only transfected with the tag protein. This method can not only increase the expression of the decoy protein and increase the success rate of the experiment, but the cost of the tag antibody is low and the affinity is strong, but after all, it is not the expression level in its physiological state, which may be different from the actual results, and it is necessary to construct a vector, which is more suitable for the situation where the expression level of the decoy protein is low or there is no IP antibody;
2) The second is to select the control IgG antibody of IP antibody. In this way, the expression of the bait protein is in a natural state, closer to the real situation, but correspondingly, the expression level may be low and the success rate may not be high. At the same time, IP-level antibodies are required;
3) The third method is to use cells with knockout bait protein as control samples. In this case, the experimental group is also under natural conditions, with high reliability, but the period required for the experiment is high, and knockout verification is required.
1. Can the sample lysate in the CoIP kit be replaced by WB lysate?
No, the lysate of CoIP generally does not contain SDS. SDS is a powerful detergent that can destroy the non-covalent interaction between proteins and denature proteins, which will lead to the dissociation of the originally interacting protein complex.
2. What is the protein concentration of COIP samples after lysis?
Not less than 1mg/mL. If the protein concentration is too high, it can be diluted with pbs.
3. What is the difference between Protein A and Protein G in agarose beads? Do they need to be added?
It is generally recommended to add them. Protein A has high affinity with rabbit antibodies, and Protein G has high affinity with mouse antibodies.
4. Comparison of the addition order of antibodies, samples and magnetic beads?
The first antibody is bound to the protein first, and then the magnetic beads are added. The second antibody is incubated with the magnetic beads first, and finally the protein is added. The third antibody is added at the same time. Generally, the first antibody is not much different from the second one. If the amount of protein is too high, such as greater than 1000ug, it is recommended to use the second one, otherwise the protein will easily adsorb on the magnetic beads and reduce the yield.
5. Does the Input group need to run internal reference?
Of course, running internal parameters in the Input group can not only assist in judging the operation, but also have a prompt effect on whether the sample is degraded.
IP/CoIP Result Interpretation and FAQ
1. How to interpret CoIP results?
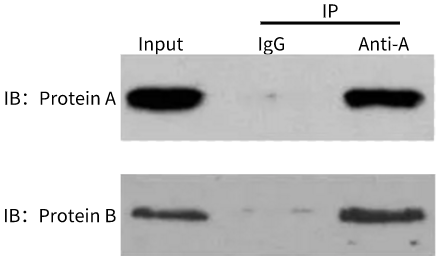
IB: Western Blotting, that is, conventional Western Blotting, is used to display the protein of interest;
IP: immunoprecipitation, this step is mainly to purify and enrich the protein of interest;
Input: Whole cell lysate containing all proteins in cells, which can be considered as a positive group. That is, the cell lysate obtained after processing the sample needs to run WB before doing IP experiments to confirm that the sample contains protein A and protein B;
Anti-A: co-immunoprecipitating antibodies to protein A;
IgG: the isotype control antibody of Anti-A, that is, the same source isotype as Anti-A. If Anti-A is a rabbit-derived IgG type, the isotype control antibody needs to be Rabbit IgG (abs20035).
Result graph analysis:
This result is to verify whether there is an interaction between protein A and protein B. This experiment is divided into two groups: Input group and IP group. The IP group is divided into IgG group (negative control group) and experimental group. WB is used to verify whether protein A and B exist in each group. From the bands in the Input group, it can be concluded that both protein A and protein B exist, which proves that there is no problem in the step of sample processing and protein extraction. The negative control IgG group and the experimental group were subjected to immunoprecipitation experiments in parallel. The results showed that there were no bands in protein A and protein B, indicating that protein A and B were not precipitated by IgG antibody, which proved that protein A and B did not bind to IgG, and ruled out the possibility of non-specific binding between protein and antibody; The experimental histones A and B both have bands, indicating that protein A was successfully precipitated by using antibodies to protein A. At the same time, protein B was also precipitated, and then it was concluded that protein A and protein B. There is an interaction between them.
2. The input group has no stripe, but the IP group has stripe
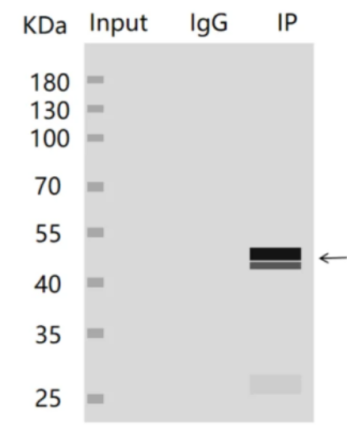
It may be due to the low abundance or degradation of the target protein in the sample, which led to the failure to successfully detect the protein in the Input group, but it was able to be detected after enrichment in the IP group. It is recommended to appropriately increase the load volume of the Input group, perform routine WB validation, and add protease inhibitors during the sample processing phase to stabilize the protein.
3. Miscellaneous tape problem
Non-specific bands should be analyzed by case. If there are miscellaneous bands in input, IP group, and IgG group, it may be non-specific binding of magnetic beads, non-specific binding caused by too high sample load, or too high antibody concentration. For specific binding, it is recommended to rinse the magnetic beads before the experiment, increase the number of washes during the experiment, and use appropriate sample and antibody concentration. If there are no miscellaneous bands in IgG group and IP group, and there are miscellaneous bands in Input, it may be because the specificity of WB antibody is not good enough, and more factors may be considered in actual experiments.
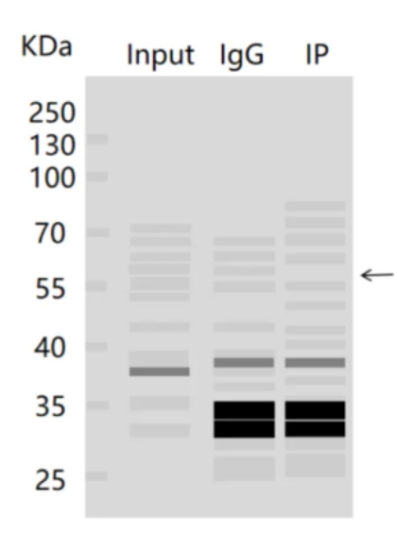
4. No bands in IP and IgG groups
Maybe because the amount of IP antibody added is small, the specificity of IP antibody is poor, or the amount of protein is too high, the Beads are covered preferentially, resulting in a small collection of IP antibodies and Beads, etc. You can give priority to increasing the amount of antibodies and optimizing the binding sequence. There is no improvement. You can only try to replace antibodies.
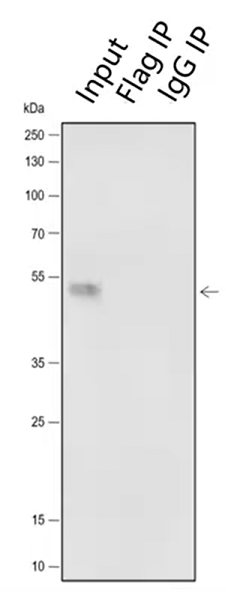
5. Light and heavy chain issues
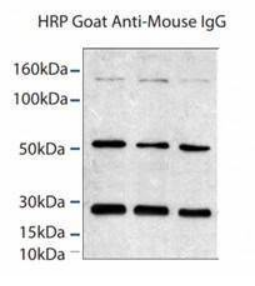
In the process of denaturation and elution, the antibody is decomposed into heavy chain 55KD and light chain molecule 25KD under the influence of high temperature and reducing agent. When the antibody used by WB is homologous to IP antibody, the secondary antibody will recognize the light and heavy chains of IP antibody. This is a common problem. When selecting antibodies, IP antibodies and WB antibodies come from different species as far as possible. Of course, even if this optimization has been done, the experimental results often still have light and heavy chains.
Note: The pictures in this article come from the Internet and are for learning reference only
Related Products
|
Item number |
Product name |
Specifications |
|
Immuno (co) precipitation (IP/CoIP) kit |
|
|
|
Immunoprecipitation (IP/CoIP) kit (magnetic bead method) |
10T/50T |
|
|
abs9146 |
PMSF (phenylmethylsulfonyl fluoride) |
5g/25g/100g |
|
abs20035 |
Rabbit IgG |
10mg |
|
abs20038 |
Mouse IgG |
1mg |
|
abs9232 |
BCA Protein Quantification Kit |
500T/2500T |
|
abs9301 |
Preformed glue (10%, 10wells) |
5 tablets/box |
|
abs924 |
Pre-stained protein marker, 10-180kDa |
250uL/250uL × 2 |
|
abs950 |
10 * Electrotransfer fluid |
100mL/1L |
|
abs954 |
WB-specific primary and secondary antibody dilution |
100mL |
|
abs961 |
10 × PBS buffer |
500mL |
|
abs952 |
TBST (10 ×) |
500mL × 2 |
|
abs920 |
ECL Chemiluminescence Assay Kit |
250mL × 2 |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. Email: worldwide@absin.net |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
