- Cart 0
- English
Cellular reactive oxygen species (ROS) assay strategy
July 11, 2025
Clicks:964
Reactive oxygen species (ROS) are by-products of cellular metabolism, including superoxide anion radicals, hydrogen peroxide, hydroxyl radicals, etc. They play a role in cell signaling, but overproduction can lead to cell damage and has been linked to a variety of diseases. Current detection methods for ROS include fluorescence staining, chemiluminescence, electron paramagnetic resonance (EPR/ESR), electrochemical biosensors, chromatography, spectrophotometry, and fluorescent protein-based methods (Table 1).
Table 1 Common detection methods for ROS
|
ROS detection method |
Description of the method |
|
Fluorescent staining method |
A variety of ROS can be detected using a specific fluorescent probe such as DCFH-DA, which is not fluorescent by itself, and is hydrolyzed by esterases to form DCFH upon entering the cell, followed by oxidation by ROS to produce fluorescent DCF |
|
Chemiluminescence method |
ROS is quantified using the luminescence signal produced by the reaction of certain chemicals with ROS |
|
Electron Paramagnetic Resonance (EPR/ESR) |
A method for the direct detection of free radicals, suitable for the detection of a variety of ROS, including hydroxyl radicals |
|
Electrochemical biosensors |
ROS is detected by electrochemical method with high sensitivity and selectivity |
|
chromatography |
Specific ROS or its metabolites are detected by chromatographic separation techniques |
|
Spectrophotometry |
ROS is detected using a specific spectrophotometer, such as the superoxide anion by detecting the reduction of nitrotetrazolium blue (NBT). |
|
Fluorescent protein-based methods |
Genetic engineering was used to fuse fluorescent proteins with ROS-sensitive peptides, and ROS was detected by fluorescence changes |
Measuring ROS levels is essential to understand cellular damage caused by cellular physiological functions and environmental factors. Today, Xiaoai will focus on the principle and specific operation steps of fluorescent staining to detect intracellular ROS.
Reactive oxygen species detection strategy
1. Choose the right probe
DCFH-DA: This is a commonly used fluorescent probe that quantifies intracellular ROS levels by detecting the fluorescence intensity of its oxidation product, DCF. DCFH-DA itself is non-fluorescent, can freely cross the cell membrane, is hydrolyzed by esterases in the cell to form DCFH, and then oxidized to form DCF, and its fluorescence intensity is proportional to the level of ROS.
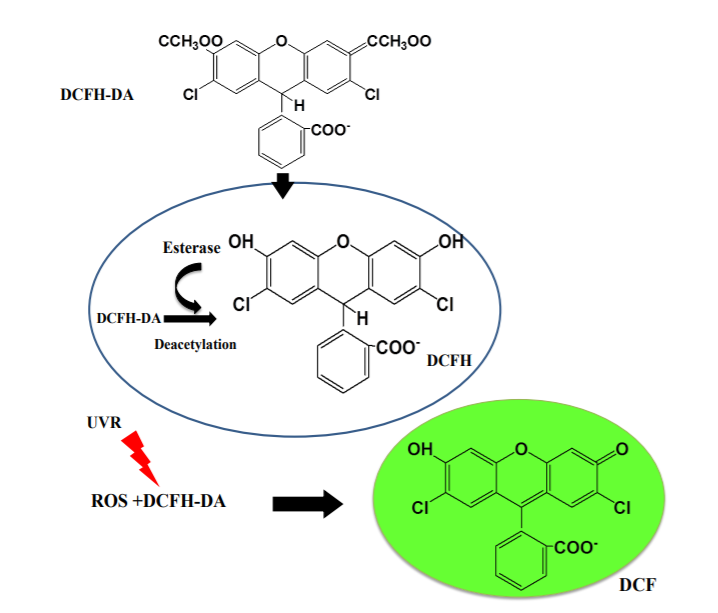
Fig.1 Mechanism of action of DCFH-DA probe in cells[1]
2. Experimental procedure (take abs580232, reactive oxygen species ROS detection kit as an example)
1. Load the ROS probe
1) In situ loading probe (for adherent cells only)
(1) Cell preparation: Plate the cells one day before the test to ensure that the number of cells is less than 5×10 at the time of detection5/mL。
(2) Drug induction: remove the cell culture medium, add the drug treatment diluted with serum-free medium, and incubate in the cell culture incubator at 37 °C in the dark, the actual induction time is determined by the drug characteristics and cell type.
(3) (optional) positive control: first dilute the positive control (abs580232, Rosup, 100mM) with serum-free medium to the usual working concentration of 100 μM, add cells, and incubate at 37 °C in the dark for 30min-4h to increase the level of reactive oxygen species, which is different in different cell types. For example, HeLa cells need to be incubated for 30-60 minutes, and MRC5 human embryonic fibroblasts need to be incubated for 90 minutes.
(4) ROS probe preparation: Dilute DCFH-DA with serum-free culture medium at 1:1000 before probe loading to make its final concentration 10 μM.
(5) ROS probe loading: Suction the treated drug and add an appropriate volume of diluted DCFH-DA working solution. The volume added should cover the cells sufficiently. For example: a 6-well plate is usually not less than 1 mL, and for a 96-well plate it is usually not less than 100 μL. Incubate at 37°C in the cell culture incubator in the dark for 30min.
(6) Cell washing: Wash the cells 1-2 times with serum-free culture medium to fully remove the DCFH-DA that has not entered the cells.
2) Loading probes after collecting cells (suitable for adherent and suspension cells)
(1) Cell preparation: Cells are cultured according to standard methods, and the state of cells for detection must be ensured. Follow the appropriate method to wash and collect a sufficient amount of cells.
(2) Drug induction: The collected cells are suspended in an appropriate amount of diluted drug and incubated in a cell culture incubator at 37°C in the dark, and the actual induction time is determined by the drug characteristics and cell type.
(3) (Optional) Positive control: Dilute the positive control (abs580232, Rosup, 100mM) to the usual working concentration of 100 μM with serum-free medium, add cells, and incubate at 37°C in the dark for 30min-4h to increase the level of reactive oxygen species, there are differences in different cell types. For example, HeLa cells need to be incubated for 30-60 minutes, and MRC5 human embryonic fibroblasts need to be incubated for 90 minutes.
(4) ROS probe preparation: Before probe loading, dilute DCFH-DA with serum-free culture medium at 1:1000 to make it a final concentration of 10 μM.
(5) Probe loading: remove the intracellular drug, centrifuge to collect the cells, and add the diluted probe to make its cell density 1.0×106-2.0×107。 Note: Cell density needs to be adjusted according to the subsequent assay system, assay method, and total assay. For example, for flow cytometry, the number of cells in a single tube assay is not less than 104, not more than 106。
(6) Cell washing: Wash cells 1-2 times with serum-free cell culture medium to fully remove DCFH-DA that has not entered the cells.
2. Fluorescence microscope detection
1) Adherent cells or living tissues can be directly observed under a fluorescence microscope; For suspension growth cells, drop 25-50 μL of cell suspension onto a microslide and cover with a coverslip.
2) Under the fluorescence microscope, the FITC filter was used to observe the fluorescence, and the background was removed to observe the change of fluorescence.
3. Flow cytometry analysis
1) For adherent growth cells, trypsinize to prepare a single-cell suspension; For suspension growth cells, collect the cells directly. Resuspend cells with 0.5-1 mL PBS (0.5-1×105/mL)。
2) Select FL1 or BL1 channel of flow cytometry, excite at 488 nm, and measure the emission at 530 nm, the cells should be divided into two subsets: ROS-negative cells have only very low fluorescence intensity, and ROS-positive cells have strong green fluorescence.
Application of DCFH-DA in ROS detection
Gao[2]The standard fluorescent probe 2′,7′-dichlorodihydrofluorescent yellow diacetic acid (abs42197174, DCFH-DA) was used to determine ROS in 4T1 cells after liposome treatment. The specific operation process is as follows:
1) Seed 4T1 cells in 35 mm Petri dishes at a density of 8×104Cells/wells.
2) After the cell culture reaches 70–80% confluence for 24 hours, liposomes (1 mL, 1 mg/mL) are added to the culture medium. After 6 h of recipe treatment, cells are washed three times with PBS.
3) Stained with DCFH-DA (abs42197174, 10 μM), after 30 min of incubation, cells were washed three times with PBS, and then CLSM imaging was performed using a confocal laser scanning microscope.

Figure 2 ROS imaging in 4T1 cells[2]
Regardless of liposome type, fucose-engineered liposomes produce more ROS in 4T1 cells than non-targeted liposomes (Figure 2), mainly because of enhanced cellular uptake. Due to DAPC peroxidation, DAPC-Fuc is more efficient than DOPC-Fuc in ROS generation.
References:
[1] Rajneesh, Pathak J, Chatterjee A, et al. Detection of Reactive Oxygen Species (ROS) in Cyanobacteria Using the Oxidant-sensing Probe 2',7'-Dichlorodihydrofluorescein Diacetate (DCFH-DA)[J]. Bio Protoc. 2017; 7(17):e2545.
[2] Gao Z, Zhang J, Hou Y, et al. Boosting the synergism between cancer ferroptosis and immunotherapy via targeted stimuli-responsive liposomes[J]. Biomaterials.2024; 305:122442.
Recommended Cell ROS Assay Products:
|
Catalog number |
Product name |
specification |
|
2,7-Dichlorodihydrofluorescent yellow diacetic acid (DCFH-DA) |
100mg |
|
|
Reactive Oxygen Species ROS Detection Kit (Green Fluorescence) |
1000T |
|
|
Reactive Oxygen Species ROS Detection Kit (Red Fluorescence) |
100-500T |
Oxidative stress related product recommendations:
|
Test items |
Item No. - Specifications |
Product name |
Detection method/range |
|
Oxidation products of DNA |
Rabbit anti-8-OHdG Polyclonal Antibody |
IHC-P |
|
|
Comet DNA Damage Analysis Kit (3-well slides) |
Nucleic acid electrophoresis |
||
|
Lipid peroxidation products |
Malondialdehyde (MDA) detection kit |
Colorimetry; 0.01mmol/L-1mmol/L |
|
|
Antioxidant enzymes |
Superoxide dismutase (SOD) assay kit |
Colorimetry; 1U/mL-30U/mL |
|
|
Catalase (CAT) test kit |
Colorimetry; 1mmol/L-100mmol/L |
||
|
Glutathione peroxidase assay kit |
Colorimetry; 0.01mmol/L-5mmol/L |
||
|
Glutathione reductase assay kit |
Colorimetry; 4umol/L-400umol/L |
||
|
Glutathione sulfurtransferase (GST) test kit |
colorimetry |
||
|
Thiodoxin oxidoreductase assay kit |
colorimetry |
||
|
Non-enzymatic antioxidants |
Glutathione test kit |
Colorimetry; 0.01mmol/L-0.5mmol/L |
|
|
Vitamin E test kit |
Colorimetry; 50umol/L-2000umol/L |
||
|
Vitamin C test kit |
Colorimetry; 0.02mmol/L-2mmol/L |
||
|
Coenzyme Q10 Assay Kit |
Colorimetry; 0.1mmol/L-10mmol/L |
||
|
Total antioxidant capacity |
Total Antioxidant Capacity (T-AOC) Assay Kit |
Colorimetry; 0.04mmol/L-4mmol/L |
|
|
Free radical scavengers |
5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) |
Electron spin resonance method |
Mitochondrial Oxidative Damage Detection Product Recommendation:
|
Catalog number |
Product name |
specification |
|
JC-1 iodide |
5mg |
|
|
Mitochondrial Respiratory Chain Complex I. Activity Assay Kit (Micro Method) |
96T |
|
|
Mitochondrial Respiratory Chain Complex II. Activity Assay Kit (Micro Method) |
96T |
|
|
Mitochondrial Respiratory Chain Complex III. Activity Assay Kit (Micro Method) |
96T |
|
|
Mitochondrial Respiratory Chain Complex IV. Activity Assay Kit (Micro Method) |
96T |
|
|
Mitochondrial Respiratory Chain Complex V. Activity Assay Kit (Micro Method) |
96T |
|
|
Comet DNA Damage Analysis Kit (3-well slides) |
15T |
|
|
Mitochondrial Permeability Transition Pore (MPTP) Assay Kit |
50T |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. Email: worldwide@absin.cn |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
