- Cart 0
- English
Caspase-Dependent Apoptosis: An Overview
May 08, 2025
Clicks:998
Introduction to the Caspase Family:
The Caspase family is a group of proteases located in the cytoplasm, playing a crucial role in programmed cell death (such as apoptosis) and inflammatory processes. Caspase is an acronym for "Cysteine-dependent Aspartate-directed proteASEs," with a cysteine-containing active site that specifically cleaves peptide bonds following aspartic acid residues in target proteins.
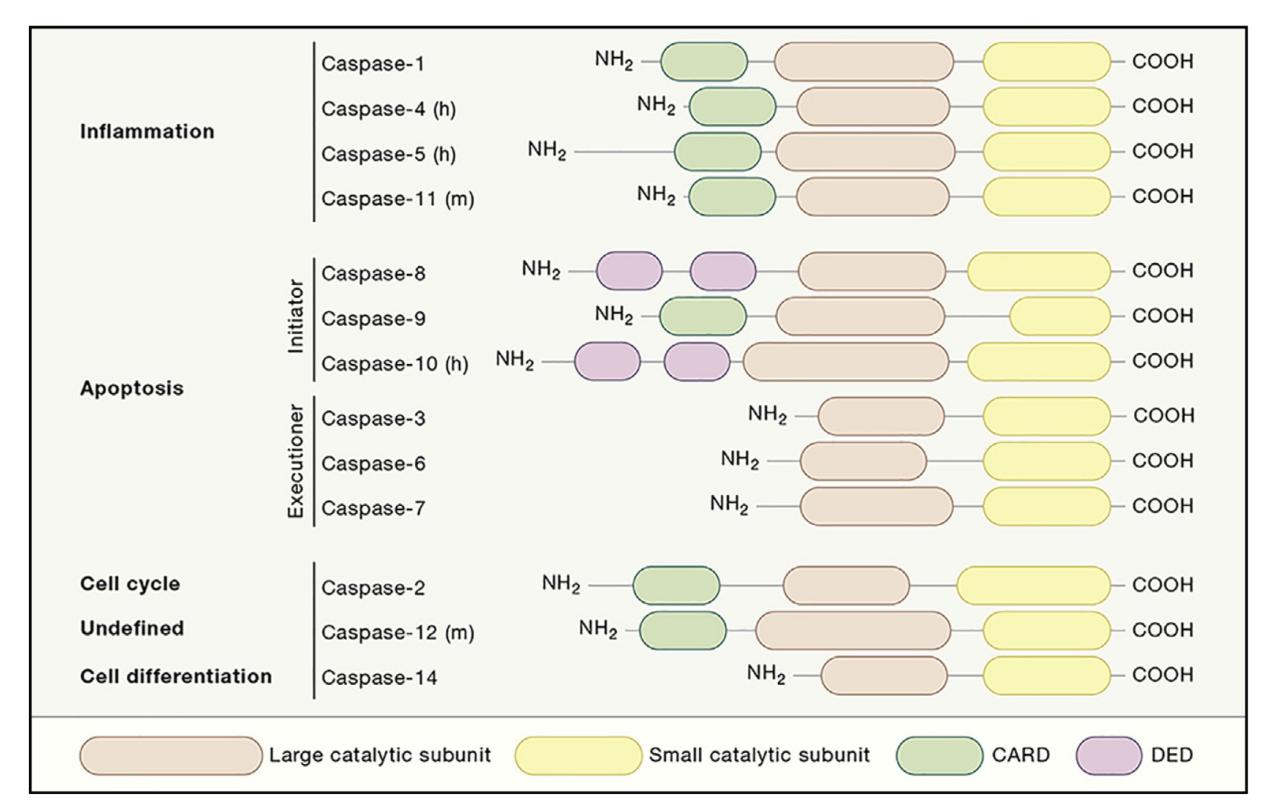
Caspase Family
Members of the Caspase Family:
Caspases can be categorized into inflammatory caspases and apoptotic caspases based on their functions:
Inflammatory caspases: These include Caspase-1, -4, -5, and -11, which primarily participate in the activation of interleukin precursors during inflammatory responses rather than directly in apoptosis signaling.
Apoptotic caspases: These are mainly involved in apoptosis and can be further divided into initiator caspases, such as caspase-2, -8, -9, -10, which are responsible for cleaving the precursors of effector caspases to produce active caspases.
Effector caspases: Caspase-3, -6, -7, which are responsible for cleaving structural and regulatory proteins in the nucleus and cytoplasm, leading to their inactivation or activation.
Characteristics of Apoptosis-Related Caspases:
Activation Mechanism
Self-cleavage activation: Many caspases exist in an inactive precursor form and require specific stimuli or interactions with other molecules to undergo self-cleavage and activation. For example, caspase-8 and caspase-9 are activated when they bind to specific adapter proteins to form complexes.
Cascading reaction: Once initiator caspases are activated, they further activate more effector caspases, creating a signal-amplifying cascade. This mechanism ensures that apoptosis is irreversible.
Substrate Specificity
Caspases exhibit high specificity for their substrates, typically recognizing aspartic acid residues in tetrapeptide sequences and cleaving at that position. Different caspases prefer different cleavage sites, determining their specific functions. Effector caspases can cleave a variety of intracellular proteins, such as lamin and poly (ADP-ribose) polymerase (PARP), leading to characteristic changes like cytoskeleton destruction, DNA fragmentation, and cell membrane alterations.
Structural Characteristics
All caspases consist of a large catalytic subunit and a small regulatory subunit, which together form a heterodimer. Each subunit is composed of multiple functional domains, such as CARD (Caspase Recruitment Domain) and DD (Death Domain), which are involved in caspase activation and interactions with other proteins.
Regulatory Mechanisms
Under normal conditions, caspases exist in cells as precursors and are strictly regulated. Only when cells receive specific death signals is the inhibition lifted, allowing caspase activation. Some inhibitors, such as IAPs (Inhibitor of Apoptosis Proteins), can directly bind to caspases to prevent their activity and thus inhibit apoptosis.
Activation of Caspases
The activation of caspases can be divided into homotypic activation and heterotypic activation, both of which play a vital role in apoptosis. The following is a detailed explanation of these two activation methods and their characteristics.
1. Homotypic Activation
Homotypic activation is the process by which caspase molecules interact to form dimers or multimers, thereby facilitating self-cleavage and activation. This process typically involves interactions between procaspases. In homotypic activation, an unactivated procaspase interacts with another procaspase through specific domains (such as CARD or DD) to form homodimers or multimeric complexes. This oligomeric state induces a conformational change in the procaspase, enabling self-cleavage, removal of inhibitory regions, exposure of the active site, and ultimately the formation of a mature caspase with catalytic activity.
For example, Caspase-9: In the mitochondrial pathway, after cytochrome c is released from mitochondria into the cytoplasm, it binds to Apaf-1 to form the apoptosome. This complex recruits multiple procaspase-9 molecules, promoting their homotypic activation.
Caspase-8: In the extrinsic pathway, when death receptors bind to their ligands and recruit adapter proteins to form the DISC (Death Inducing Signaling Complex), procaspase-8 undergoes homotypic activation through interactions within the DISC.
2. Heterotypic Activation
Heterotypic activation refers to the promotion of each other's activation through interactions between different types of proteins or caspase family members. This type of activation typically requires the involvement of other auxiliary factors or adapter proteins. In heterotypic activation, a procaspase interacts with non-caspase proteins, which may act as activators or scaffolds to help procaspase positioning and conformational changes. For instance, certain adapter proteins can provide specific binding interfaces to promote the aggregation and conformational changes of procaspases, thereby facilitating their self-cleavage and activation.
For example, Caspase-3 and Caspase-7: Although primarily activated directly by initiator caspases as effector caspases, they sometimes require the assistance of specific scaffold proteins or other regulatory molecules for complete activation.
Bid protein: In the intrinsic pathway, caspase-8 not only directly activates downstream effector caspases but also cleaves the Bid protein to generate tBid, which migrates to mitochondria, further amplifying apoptotic signals and indirectly promoting the activation of other caspases.
Homotypic activation relies on direct interactions between procaspases to form homodimers or multimers, promoting self-cleavage and activation. This is commonly seen in the activation of initiator caspases such as caspase-8 and caspase-9. Heterotypic activation involves interactions between different proteins, with some proteins acting as activators or scaffolds to assist in procaspase positioning, aggregation, and conformational changes. This is commonly observed in complex multi-step apoptosis signaling pathways.
Caspase-Mediated Apoptotic Pathways
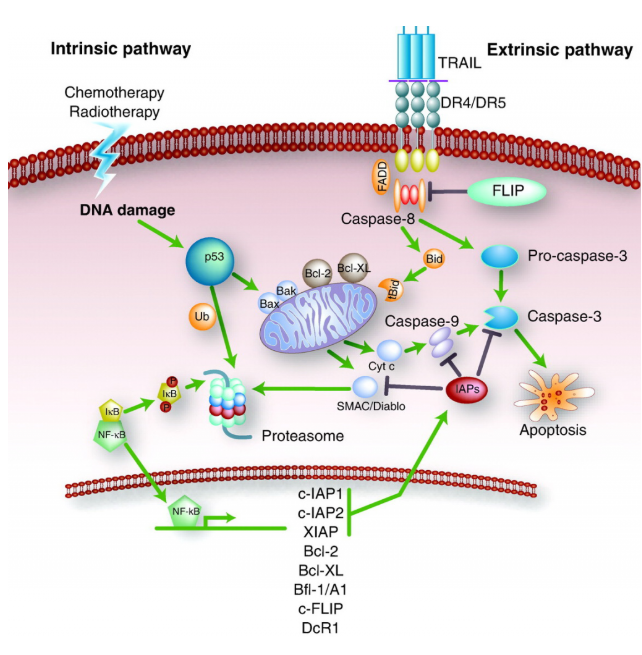
Apoptotic Pathways
Caspase-mediated apoptotic pathways are an important mechanism of programmed cell death, primarily divided into two categories: the extrinsic (death receptor) pathway and the intrinsic (mitochondrial) pathway. These pathways execute apoptosis by activating a series of caspase proteases.
1. Extrinsic (Death Receptor) Pathway
Initiation: This pathway is activated when specific ligands, such as Fas ligand or tumor necrosis factor (TNF), bind to corresponding receptors on the cell surface. These receptors are members of the TNF receptor family.
Signaling: After ligand binding, adapter proteins are recruited to form a large molecular complex called the Death Inducing Signaling Complex (DISC) near the cell membrane. This process leads to the self-cleavage and activation of initiator caspase-8.
Execution Phase: Activated caspase-8 can directly cleave and activate effector caspases such as caspase-3, -6, -7, triggering apoptosis programs. Alternatively, it can indirectly activate the mitochondrial pathway by cleaving the pro-apoptotic protein Bid, further amplifying apoptotic signals.
2. Intrinsic (Mitochondrial) Pathway
Triggering Factors: Various internal cellular stimuli, including DNA damage, oxidative stress, hypoxia, and cell cycle arrest, can activate this pathway.
Bcl-2 Family Regulation: Proteins in the Bcl-2 family play a key role in this process. Anti-apoptotic members (such as Bcl-2, Bcl-xL) inhibit apoptosis, while pro-apoptotic members (such as Bax, Bak) promote apoptosis. When pro-apoptotic signals dominate, Bax/Bak form pores in the cell membrane, increasing mitochondrial membrane permeability.
Cytochrome c Release: With increased mitochondrial membrane permeability, cytochrome c is released from mitochondria into the cytoplasm. It forms the apoptosome with other proteins, activating caspase-9.
Execution Phase: Activated caspase-9 subsequently activates downstream effector caspases such as caspase-3, -6, -7, which cleave specific substrates, leading to cellular structural changes and ultimately apoptosis.
Although these two pathways have different triggering mechanisms, they ultimately execute apoptosis through the activation of a series of caspases. Additionally, it should be noted that in some cases, these two pathways are not entirely independent but can interact and regulate each other to ensure the accurate execution of apoptosis. For example, the extrinsic pathway can indirectly influence the intrinsic pathway by activating the Bid protein. Similarly, the intrinsic pathway can also affect the efficiency and effectiveness of the extrinsic pathway. This complex interaction network ensures apoptosis as a precisely regulated biological phenomenon.
Methods for Detecting Caspases
Caspase detection methods include flow cytometry, Western Blotting (WB), Immunofluorescence (IF), Immunohistochemistry (IHC), ELISA, etc., such as:
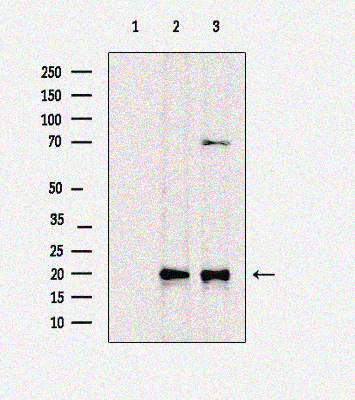
Rabbit anti-Cleaved-caspase3 Polyclonal Antibody
Cleaved caspase-3 (Asp175) p17Ab was used for Western Blotting analysis of extracts from different samples. Lane 1: Rat liver with antigen-specific peptide block. Lane 2: Mouse liver. Lane 3: Rat spleen.
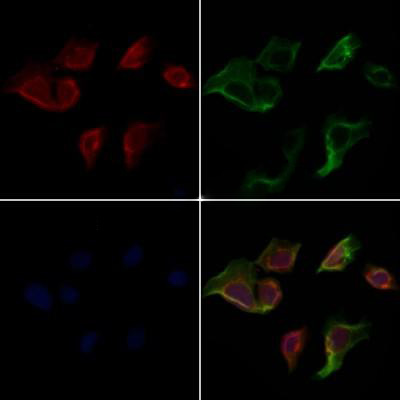
Rabbit anti-Cleaved-caspase3 Polyclonal Antibody
Hela cells were stained by IF/ICC. Samples were fixed with PFA, permeabilized in 0.1% Triton X-100, and then blocked in 10% serum at 25°C for 45 minutes. Samples were then incubated with primary antibody (1:200) and mouse anti-β-tubulin antibody (1:200) at 37°C for 1 hour. AlexaFluor594-conjugated goat anti-rabbit IgG (H+L) Ab (Red) and AlexaFluor488-conjugated goat anti-mouse IgG (H+L) Ab (Green) were used as secondary antibodies. Nuclei were counterstained with DAPI (Blue).
Related Product Recommendations
|
Product No. |
Product Name |
Specifications |
|
Rabbit anti-Caspase 9 Polyclonal Antibody |
100uL |
|
|
Rabbit anti-Caspase 10 Monoclonal Antibody(2J3 |
100uL |
|
|
Rabbit anti-Caspase-9 Polyclonal Antibody |
100uL |
|
|
Rabbit anti-Caspase-1 P10 Polyclonal Antibody |
100uL |
|
|
Rabbit anti-Caspase 3 Polyclonal Antibody |
100uL |
|
|
Rabbit anti-Caspase 3 p17 Polyclonal Antibody |
100uL |
|
|
Rabbit anti-Caspase 5 p10 Polyclonal Antibody |
100uL |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
