- Cart 0
- English
Comprehensive Guide to Apoptosis Detection
I. Overview
Apoptosis is an active, orderly form of cell death involving the activation, expression, and regulation of a series of genes. It is not a phenomenon of self-damage under pathological conditions but an active form of death to better adapt to the environment. Today, I will introduce to you six commonly used methods for detecting apoptosis: Phosphatidylserine Detection on Cell Membrane, TUNEL End Labeling Method, Mitochondrial Membrane Potential Detection, Caspase Apoptosis Core Molecule Detection, Morphological Observation, and Mitochondrial Permeability Transition Pore Detection.

Comprehensive Methods for Apoptosis Detection
II. Detection Methods
1. Phosphatidylserine Detection on Cell Membrane
Detection methods based on cellular metabolites include the Annexin V-FITC/PI Detection Method (abs50001), Annexin V-EGFP/PI Detection Method (abs50006), Annexin V-APC/PI Detection Method (abs50009), Annexin V-PE/7-AAD Detection Method (abs50007), and Annexin V-APC/7-AAD Detection Method (abs50008). Today, I will focus on the Annexin V-FITC/PI Detection Method.
(1) Detection Principle

(2) Applicable Samples: Adherent cells, suspension cells
(3) Equipment: Flow cytometer/fluorescence microscope
(4) Advantages: Annexin is one of the most sensitive indicators; qualitative and quantitative
(5) Annexin V Apoptosis Detection Results
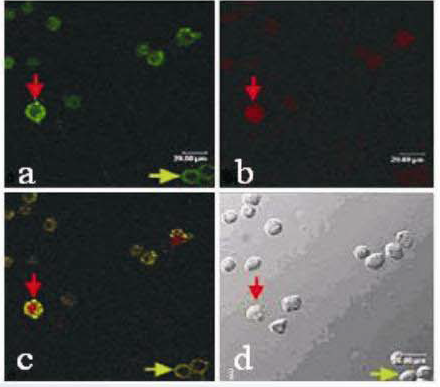
Under a fluorescence microscope, dual staining with Annexin V-FITC & PI can distinguish normal cells, early apoptotic cells, late apoptotic cells, and necrotic cells. As shown in the figure above, a, b, c are apoptotic cells under a fluorescence microscope, where a, b are singly stained with Annexin V-FITC and PI, respectively, and c is doubly stained with Annexin V-FITC & PI; d is apoptotic cells under an optical microscope (red arrows indicate late apoptotic cells, and green arrows indicate early apoptotic cells).
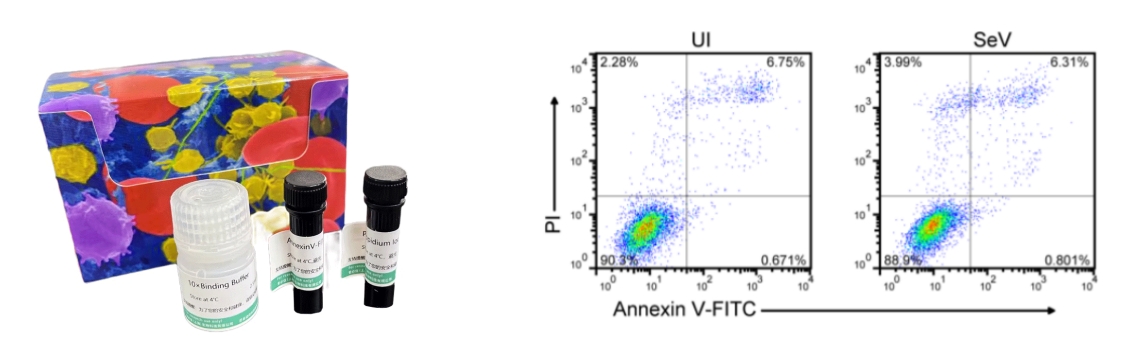
The effect of the customer using the Annexin V-FITC/PI Apoptosis Detection Kit (abs50001) is shown in the figure above. Publication name: Serine metabolism antagonizes antiviral innate immunity by preventing ATP6V0d2-mediated YAP lysosomal degradation Journal: Cell Metabolism Impact Factor: 27.287
(6) Precautions for Annexin V Apoptosis Detection
A. It is recommended to not use trypsin containing EDTA to digest adherent cells;
B. It is recommended to collect suspended cells from the culture medium separately before trypsin treatment and store them in 2% BSA;
C. The reagent should be centrifuged at low speed before use to avoid liquid accumulation on the cap and wall of the tube;
D. Annexin V-FITC and PI are photosensitive substances. Please pay attention to light protection during operation. Try to perform the procedure in the dark. During the incubation stage, wrap the container with aluminum foil or place it in a drawer. Observe the cells under a microscope in a dark room after labeling;
E. The entire operation process should be as gentle as possible. Avoid blowing the cells hard. Try to operate at 4℃ to avoid affecting cell status;
F. In the last step of cell washing, please try to discard the supernatant completely to avoid residual PBS, which may affect the experimental results;
G. To prevent fluorescence decay, it is advisable to perform flow cytometry detection within 1 hour;
H. Prolonged PI staining may cause an increased detection rate of apoptosis. It is recommended to first perform Annexin V-FITC staining, and PI staining can be added at least 5 minutes before loading.
2. TUNEL End Labeling Method
Detection methods based on TUNEL ends include the TUNEL-Biotin Detection Method (abs50022), TUNEL-488 Detection Method (abs50047), and TUNEL-594 Detection Method (abs50058). Today, I will focus on the TUNEL-488 Detection Method.
(1) Detection Principle
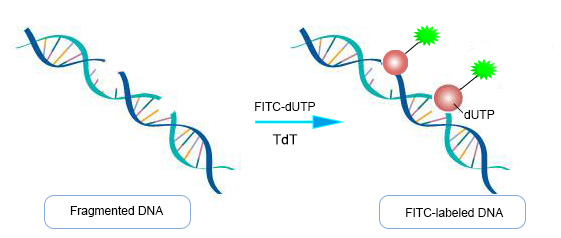
Principle of TUNEL End Labeling Method Detection
About TUNEL end labeling, when the genome DNA is double-stranded or single-stranded broken, a large number of sticky 3'-OH ends are produced, which can be combined with 488/Cy-dUTP under the catalysis of terminal deoxynucleotidyl transferase (TdT), thus allowing direct detection of apoptotic cells by fluorescence microscopy or flow cytometry.
(2) Applicable Samples: Adherent cells, suspension cells, cell smears, paraffin sections, frozen sections
(3) Equipment: Flow cytometer, optical/confocal/fluorescence microscope, fluorescence microplate reader, etc.
(4) Advantages: Simple operation, accurate qualitative results
(5) TUNEL Apoptosis Detection FAQ
A, What is the difference between the red and green fluorescence kits and the biotin-labeled kits?
The biggest difference lies in the different equipment they are compatible with. The biotin-labeled kits are compatible with optical microscopes, so when staining cell nuclei, biotin-labeled kits are generally compatible with hematoxylin or methyl green, while fluorescent kits are generally compatible with DAPI, but fluorescent kits have shorter experimental times, easier operation, and easier observation of results.
B, What is the role of positive and negative controls?
Positive control is used to verify if this experiment and kit are problematic, and negative control is to exclude non-specific staining caused by cell apoptosis itself, operation process, or dye reasons, and is also used to adjust the exposure strength of the shot. Generally, only one positive and one negative control are needed, and multiple tissues can also set up multiple negative controls.
C, Non-specific labeling (false positives)?
a, The level of nuclease or polymerase activity in cells or tissues is relatively high (such as smooth muscle), DNA is cut, and false positives are easy to occur. It is recommended to immediately and fully fix cells or tissues after they are taken out to stop the activity of these enzymes, and setting up negative controls helps to judge;
b, Endogenous peroxidase affects, it is recommended to extend the blocking time and increase the concentration of hydrogen peroxide for tissues with many blood cells such as liver, kidney;
c, The concentration of fixative is too high or too low, resulting in poor fixation effect in the center of the tissue, causing central cells to autolyze and DNA chains to break irregularly, producing false positives. It is recommended to use 4% paraformaldehyde;
d, The reaction time of TdT enzyme is too long or the reaction solution evaporates or leaks during the Tunel reaction process, and the surface of the cells or tissues cannot remain moist. It is recommended to pay attention to controlling the reaction time and ensure that the TdT enzyme reaction solution can cover the sample;
e, Paraffin, fat, blood, body fluids, etc. are more likely to bind with dyes, so the tissue section production process should be kept clean and dewaxing should be thorough;
f, Some reagents or cells have spontaneous fluorescence, and it is necessary to avoid the color of spontaneous fluorescence when selecting dyes.
D, No fluorescence staining?
a, Insufficient fixation. The fixative of choice is 4% paraformaldehyde, prepared fresh as needed. Ethanol is not recommended, as ethanol fixative has weak penetration of tissue, affecting TUNEL labeling efficiency;
b, Insufficient permeability of the cell membrane and nuclear membrane, TdT enzyme failed to reach the nucleus. For cells and frozen sections, 2% Triton X-100 solution can be used for 5-30 minutes, paraffin sections are recommended to use proteinase K, 37℃ for 30 minutes; different cells and tissues require slightly different permeation times, too long is easy to peel off, adjust appropriately;
c, Extend the labeling time to 2 hours, and appropriately increase the amount of TdT enzyme and dUTP;
d, Ensure that the experimental subject cells have apoptosis. Prepare a DNase I treated positive control to verify whether the TdT enzyme reaction is proceeding correctly.
E, High fluorescence background?
a, Mycoplasma contamination: Mycoplasma staining detection kits can be used for verification;
b, The reaction time of TdT enzyme is too long, the TdT enzyme provided by the kit can be diluted 2-5 times according to the instructions, and the diluted TdT enzyme is only for use on the same day;
c, DAB incubation time is too long, reduce DAB staining time;
d, Cells in a state of rapid division and proliferation may also have DNA breaks in the nucleus. It is recommended to sample and test during the non-proliferative period;
e, Some reagents or cells have spontaneous fluorescence, and it is necessary to avoid the color of spontaneous fluorescence when selecting dyes;
f, Non-specific binding of Biotin-X-dUTP, after the TdT enzyme reaction, wash three times with PBS containing 0.1% Triton X-100 and 1mg/mL BSA.
F, Low labeling rate?
a, Samples fixed with ethanol, methanol, or formaldehyde (most formaldehyde purchased on the market contains methanol) have lower labeling efficiency (because during fixation, chromatin fails to cross-link with proteins and is lost during operation), use the recommended fixative;
b, Excessive fixation time, leading to excessive cross-linking, reduce fixation time;
c, If adherent cells are induced to apoptosis with drugs, the cells will shrink and the adhesion force will decrease, causing apoptotic cells to easily fall off. It is recommended to be gentle in operation, preventing apoptotic cells from being washed away during washing. The subsequent entire operation also needs to be gentle.
3. Caspase Apoptosis Core Molecule Detection
Detection methods based on caspase apoptosis core molecules include Caspase 1 Activity Detection Method (abs50023), Caspase 2 Activity Detection Method (abs50024), Caspase 3/7 Activity Detection Method (abs50025), Caspase 4 Activity Detection Method (abs50026), and Caspase 6 Activity Detection Method (abs50027). Today, I will focus on the Caspase 3/7 Activity Detection Method.
(1) Detection Principle
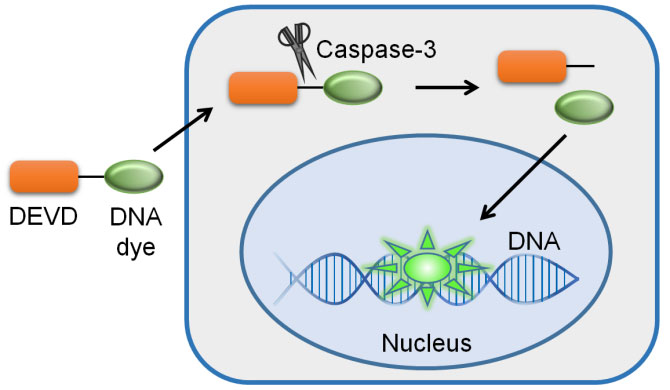
Principle diagram of Caspase-3 activity detection kit
Based on Caspase 3/7 can catalyze the substrate Ac-DEVD-pNA to produce yellow pNA (p-nitroaniline), thus caspase 3/7 activity can be detected by measuring the absorbance. pNA has a strong absorption near 405nm.
(2) Applicable Samples: Adherent cells, suspension cells, tissue samples
(3) Equipment: Flow cytometer, confocal/fluorescence microscope, fluorescence microplate reader, etc.
(4) Advantages: High sensitivity, strong specificity, no cross-reaction with other known Caspases
4. Mitochondrial Membrane Potential Detection
Detection methods based on mitochondrial membrane potential include the Mitochondrial Membrane Potential Detection Kit (JC-10) (abs50017) and the Mitochondrial Membrane Potential Detection Kit (JC-1) (abs50016).
(1) Detection Principle
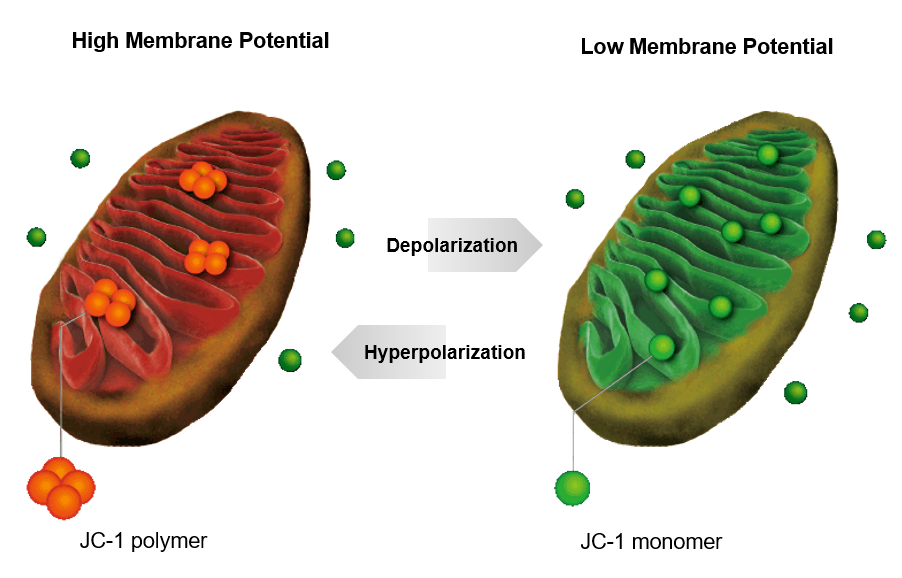
Principle diagram of Mitochondrial Membrane Potential Detection Kit (JC-10)
The decrease in mitochondrial transmembrane potential (Δψm) is the earliest event in the apoptotic cascade, and the mitochondrial membrane potential can be tracked using dyes.
(2) Applicable Samples: Adherent cells, suspension cells, purified mitochondria
(3) Equipment: Flow cytometer, confocal/fluorescence microscope, fluorescence microplate reader, etc.
(4) JC-1 Apoptosis Detection Results

Fluorescence images of JC-1-stained chondrocytes
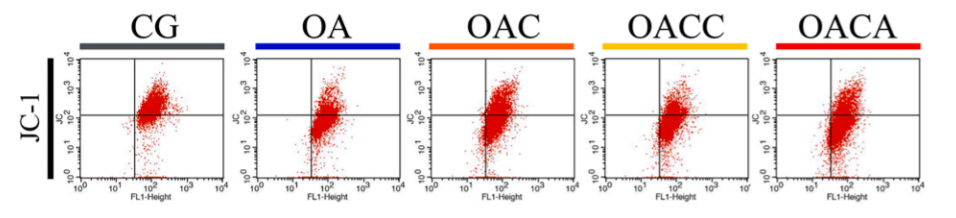
Flow cytometry results of JC-1-stained chondrocytes
The effect of the customer using the Mitochondrial Membrane Potential Detection Kit (JC-1) (abs50016) is shown in the figure above. Publication name: Curcumin exerts chondroprotective effects against osteoarthritis by promoting AMPK/PINK1/Parkin-mediated mitophagy Journal: Biomedicine & Pharmacotherapy Impact Factor: 7.18
5. Mitochondrial Membrane Permeability Transition Pore Detection
Detection methods based on mitochondrial membrane permeability transition pores include the Mitochondrial Permeability Transition Pore (MPTP) Detection Kit (abs50064).
(1) Detection Principle
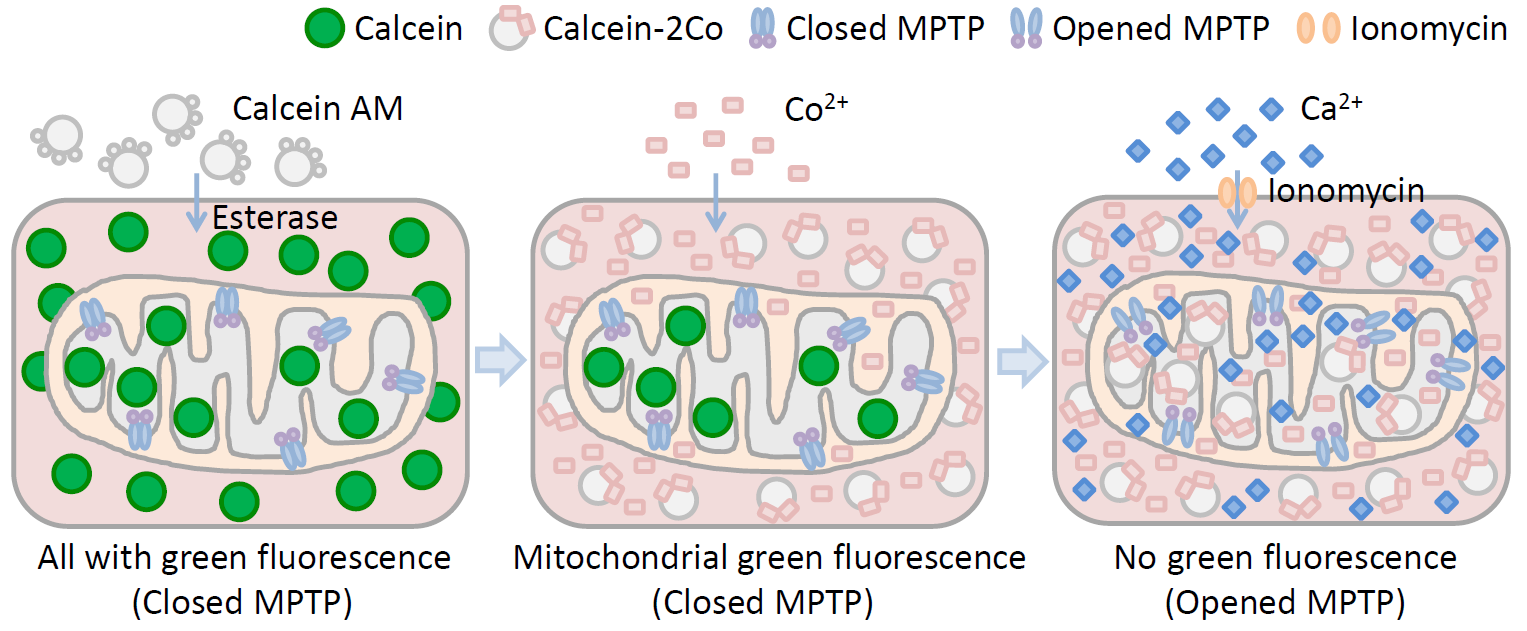
Principle diagram of Mitochondrial Permeability Transition Pore (MPTP) Detection
When cells undergo apoptosis and pathological death, the permeability of the mitochondrial membrane potential transition pore changes, Ca2+ overload, oxidation of mitochondrial glutathione, increased levels of reactive oxygen species, including subsequent release of cytochrome C, and decrease in mitochondrial membrane potential all lead to the activation of the mitochondrial permeability transition pore.
(2) Applicable Samples: Adherent cells, suspension cells
(3) Equipment: Flow cytometer, confocal/fluorescence microscope, fluorescence microplate reader, etc.
(4) Dye: Calcein AM
6. Morphological Observation
Detection methods based on cellular morphology observation include fluorescence microscopy/optical microscopy observation methods {DAPI staining method, Hoechest staining method, AO staining method, HE staining method}, and transmission electron microscopy observation methods.
(1) Fluorescence Microscopy/Optical Microscopy Observation Method
A. Detection Principle
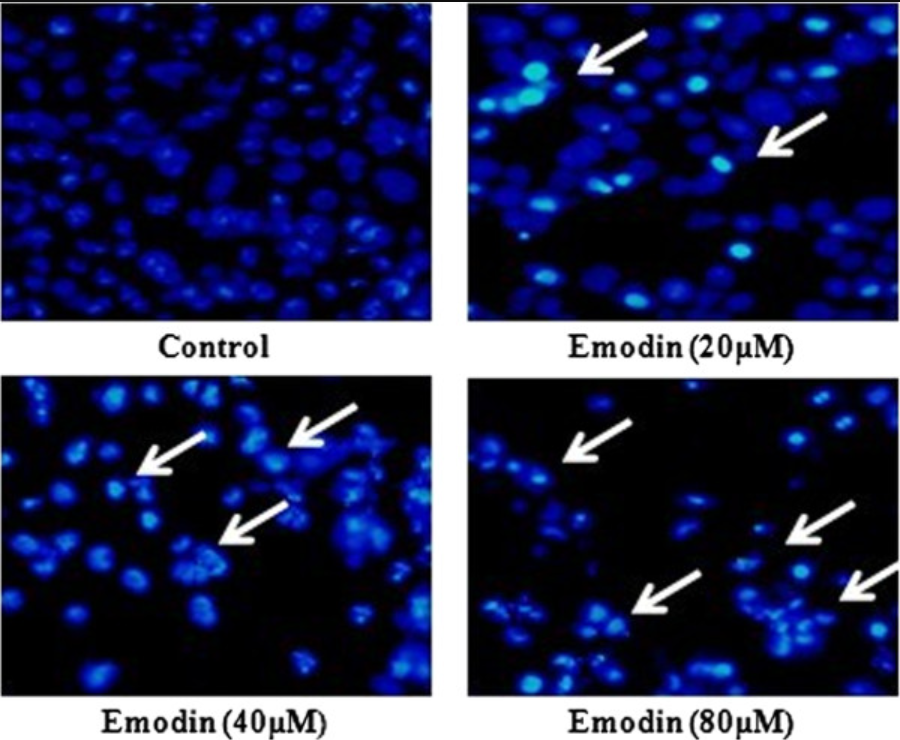
Emodin induces apoptosis of human cervical cancer hela cells via intrinsic mitochondrial and extrinsic death receptor pathway. Cancer cell international. 13. 71. 10.1186/1475-2867-13-71.
With the corresponding staining methods, directly observe the changes in cell morphology. Common fluorescence dyes include Hoechest 33342, acridine orange (AO), DAPI, etc.; chemical dyes include hematoxylin-eosin (HE), Giemsa (Giemsa) or Wright (Wright)
B. Sample Types: Adherent cells, suspension cells, cell smears, paraffin sections, frozen sections
C. Advantages: Simple operation, low cost
D. Disadvantages: Highly subjective, poor qualitative and quantitative results, rarely used alone
(2) Fluorescence Microscopy/Optical Microscopy Observation Method
A. Detection Principle
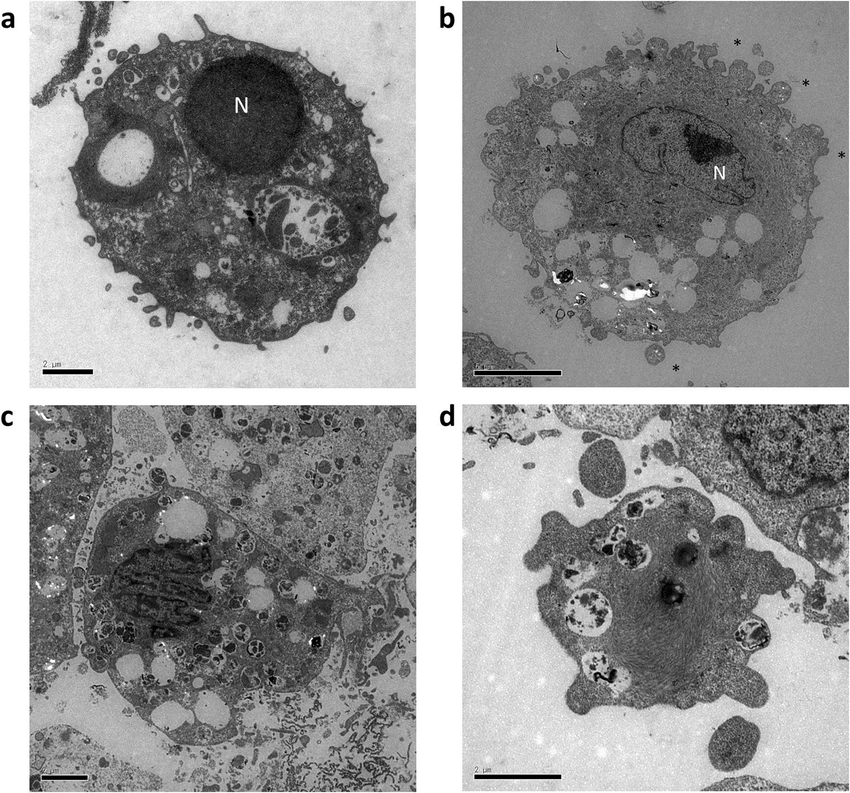
Hydrostatic pressure can induce apoptosis of the skin. Scientific Reports. 10. 10.1038/s41598-020-74695-5.
When apoptosis occurs, certain morphological characteristics appear. Under an electron microscope, apoptotic cells show condensed chromatin, nuclear condensation, nuclear fragmentation, cytoplasmic shrinkage, and the appearance of apoptotic bodies with bubble-like phenomena on the cell membrane.
B. Sample Types: Adherent cells, suspension cells, cell smears, paraffin sections, frozen sections
C. Advantages: Classic, reliable, gold standard
D. Disadvantages: High cost, time-consuming, cumbersome steps, non-quantitative, subjectivity in judgment, can only observe a small area at a time.
Recommended by Absin
|
货号 |
品名 |
规格 |
|
Annexin V-FITC/PI Apoptosis Detection Kit |
25T/50T/100T |
|
|
Annexin V-EGFP/PI Apoptosis Analysis Kit |
20T/50T |
|
|
Annexin V-APC/PI Apoptosis Analysis Kit |
25T/100T |
|
|
Annexin V-PE/7-AAD Apoptosis Analysis Kit |
25T/100T |
|
|
Annexin V-APC/7-AAD Apoptosis Analysis Kit |
25T/100T |
|
|
TUNEL Assay Apoptosis Detection Kit |
20T/50T |
|
|
TUNEL Assay Apoptosis Detection Kit(red fluorescence) |
20T/50T |
|
|
Caspase 1 Activity Assay Kit |
20T/100T |
|
|
Caspase 2 Activity Assay Kit |
20T/100T |
|
|
Caspase 3/7 Activity Assay Kit |
20T/100T |
|
|
Caspase 4 Activity Assay Kit |
20T/100T |
|
|
Caspase 6 Activity Assay Kit |
20T/100T |
|
|
Mitochondrial membrane potential detection kit (JC-1) |
100T |
|
|
Mitochondrial membrane potential detection kit (jc-10) |
100T |
|
|
Mitochondrial permeability transition pore (MPTP) assay kit |
50T/100T |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
April 22, 2025
Clicks:168
