- Cart 0
- English
3D Tumor Spheroid Culture Solutions
April 03, 2025
Clicks:1025
Overview:
Life originates in a 3D environment. Therefore, although traditional 2D cell culture methods can ensure cell growth and physiological responses to external stimuli, the significant difference from the actual living environment limits most physiological functions. For example, in a 2D culture environment, cell-cell interactions are limited to the XY axis, representing interactions between cell layers, lacking the influence of the Z-axis. Consequently, there is no gradient penetration of nutrients, oxygen, and external stimuli (e.g., drug treatment). In contrast, cells in a 3D culture environment can form spheroids, which can reflect the mechanical forces between cells along the Z-axis and the gradient penetration of various substances. Compared to 2D cell layers, the difference from in vivo conditions is greatly reduced, thereby improving the accuracy of in vitro cell experiments. Utilizing in vitro cultured tumor cells for high-throughput screening of new and effective drugs is crucial for saving cancer patients.
In this article, we will explore the process of tumor spheroid culture: using breast cancer cells MDA-MB-231 and MCF-7 as examples to prepare 3D multicellular tumor spheroids and characterize them in detail using an inverted microscope, confocal laser scanning microscope, and environmental scanning electron microscope.
Experimental Procedures:
I. Preparations Before the Experiment
1. Take 12 mL of DMEM (abs9483) and RPMI 1640 (abs9484) complete culture medium (containing 10% FBS (abs972), same below) in advance 24 hours and place them in a 50 mL centrifuge tube, then store in a 4°C refrigerator for precooling;
2. Take out the aliquoted Matrigel matrix gel (abs9490) from -20°C and place it in a 4°C environment 24 hours in advance to allow it to melt into a liquid state;
3. Place sterile 1 mL pipette tips into a sterile 50 mL centrifuge tube and store in a -20°C freezer for precooling.
II. Agarose Coating of 96-Well Plates
1. Accurately measure 6 mL of RPMI 1640 culture medium (or DMEM culture medium) into two 10 mL glass syringe bottles, add 90 mg of agarose(abs44056213), seal the bottles, and place them in a water bath at 80°C to dissolve for 30 minutes;
2. After heating, place the syringe bottles in an autoclave and sterilize at 115°C for 30 minutes;
3. After sterilization, quickly remove the syringe bottles and place them in a laminar flow hood. Pour the agarose solution from the syringe bottles into a sterile trough, and use a multichannel pipette to add 60 μL per well into a 96-well plate.
Note: Since agarose solution solidifies at room temperature, it is essential to quickly transfer the agarose solution to the laminar flow hood and add it to the 96-well plate after removing it from the autoclave.
Additionally, to ensure that the agarose does not cool during dispensing, the trough and 100 μL pipette tips should also be sterilized simultaneously.
4. After adding the solution, the 96-well plate should be kept level for approximately 30 minutes to allow the agarose in the wells to solidify.
III. Preparation of Cell Suspension with Matrigel Matrix Gel
1. Take MDA-MB-231 cells (or MCF-7 cells) in the logarithmic growth phase, digest with trypsin(abs47014938), count the cells, and adjust the cell suspension concentration to 2.0×105cells/mL using RPMI 1640 complete culture medium (or DMEM complete culture medium), and keep it on standby;
2. Place a beaker filled with crushed ice in the laminar flow hood after spraying it with alcohol, and take out the RPMI 1640 complete culture medium and the thawed Matrigel matrix gel from the refrigerator and place them on ice.
Note: Since Matrigel matrix gel solidifies at room temperature, it is essential to maintain a low temperature during the operation.
3. Take out the precooled pipette tips and place them in the laminar flow hood. According to the calculated volume (2.5%, v/v), use a pipette to add 300 μL of Matrigel matrix gel to 12 mL of RPMI 1640 complete culture medium, and mix quickly.
Note: Since Matrigel matrix gel solidifies at room temperature, the pipette tips used should also be precooled.
4. Add the cell suspension (approximately 600 μL) from step 1, resulting in a cell concentration of 10000 cells/mL, and mix quickly. Keep it on standby;
IV. Seeding the Cell Suspension into Agarose-Coated 96-Well Plates
1. Place the cell suspension containing Matrigel matrix gel prepared in step 4 into a trough, and use a multichannel pipette to aspirate 200 μL and add it to the 96-well plate coated with agarose;
2. Centrifuge using a microplate centrifuge (abs72035) under the conditions of 4°C, 1000×g, 10min;
Note: When centrifuging the 96-well plate, to maintain sterility, seal the edges of the plate with parafilm.
3. After centrifugation, remove the 96-well plate, take off the parafilm, spray with alcohol, and place it in the incubator for culturing. The entire culturing process is shown in Figure 1;

Figure 1
4. On days 3, 5, and 7 of culture, replace 100 μL of the culture medium in each well and observe the morphology of the tumor spheroids using an inverted microscope;

Figure 2
5. If using 3D multicellular tumor spheroids for drug assays, after 7 days of culture, remove 100 μL of the culture medium from each well using a pipette, add 100 μL of the drug solution, and then place it in the incubator for culturing, regularly observing the growth status of the tumor spheroids using an inverted microscope (Figure 2).
V. Characterization of 3D Multicellular Tumor Spheroids
1. Observation of 3D multicellular tumor spheroid morphology using an inverted microscope: Simply place the 96-well plate under the inverted microscope for observation;
2. Observation using confocal laser scanning microscopy: Carefully remove the tumor spheroids from the wells using a pipette, wash three times with PBS, fix with 4% paraformaldehyde(abs9179), and stain the nuclei with Hoechst 33258(abs47047617). After washing three times with PBS, observe under the confocal laser scanning microscope (Figure 3).

Figure 3
3. Observation using environmental scanning electron microscopy: Carefully remove the tumor spheroids from the wells using a pipette, wash three times with PBS (abs962), and observe after fixation and drying (Figure 4).
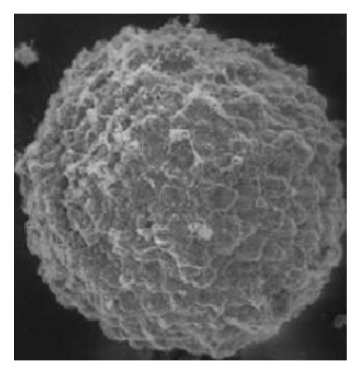
Figure 4
Common Issues:
In practice, we often encounter the issue of cells failing to form spheroids. Below, we discuss the causes of this problem in 3D cell culture and the corresponding solutions.
I. Cell Type and Characteristics:
Cell type and characteristics are key factors influencing the formation of spheroidal structures in a 3D environment. Different cell types exhibit varying self-organization and adhesion properties, which may be more suitable for forming other types of 3D structures, such as sheet-like or tubular structures.
Solution: Before conducting 3D culture experiments, it is essential to carefully select cell types suitable for self-organization. For cells that do not readily form spheroids, consider using inducers or genetic regulation methods to enhance their self-organization capabilities.
II. Lack or Inappropriateness of Extracellular Matrix (ECM):
The formation of spheroids typically requires appropriate extracellular matrix support. If the ECM components in the culture medium are insufficient or inappropriate, cells may fail to form spheroids.
Solution: Design the ECM components in the culture medium reasonably to ensure adequate support for spheroid formation. Additionally, consider adding exogenous ECM support materials, such as appropriate matrix proteins or bioactive materials.
III. Insufficient Cell Density:
Spheroid formation may require sufficient cell density to support intercellular interactions and self-organization. If the cell density is too low, it may affect the formation of spheroidal structures.
Solution: Optimize the cell seeding density to ensure an adequate number of cells for self-organization.
IV. Inappropriate Culture Conditions:
Culture conditions, such as medium composition, temperature, and oxygen concentration, may affect the ability of cells to form spheroids. Inappropriate culture conditions may hinder the self-organization of cells.
Solution: Optimize culture conditions to create an environment more conducive to cell self-organization, such as adjusting medium composition, temperature, and gas concentration.
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
