- Cart 0
- English
Three Strategies for Culturing Tumor Spheroids
March 28, 2025
Clicks:1289
Summary:
Life originates in a 3D environment, so traditional 2D cell culture methods, although capable of supporting cell growth and physiological responses to external stimuli, are significantly different from the actual living environment, leading to limitations in most physiological functions. For example, in 2D culture, cell-cell interactions are limited to the XY axis, representing interactions between cell layers, lacking the influence of the Z-axis. This results in the absence of gradient penetration of nutrients, oxygen, and external stimuli (e.g., drug treatment). In contrast, in a 3D culture environment, cells can form spheroids, which can exhibit force interactions between cells along the Z-axis and various substance penetration gradients. Compared to 2D cell layers, the differences from the in vivo environment are greatly reduced, thereby improving the accuracy of in vitro cell experiments. Utilizing in vitro cultured tumor cells for high-throughput screening of new effective drugs is crucial for saving cancer patients.
In this issue, we will explore the process of tumor spheroid culture and introduce three strategies: 3D Tumor Spheroid Agarose-Matrigel Culture Strategy, 3D Tumor Spheroid Matrigel Culture Strategy, and 3D Tumor Spheroid Ultra-Low Attachment Culture Strategy.
I. 3D Tumor Spheroid Agarose-Matrigel Culture Strategy
Using breast cancer cells MDA-MB-231 and MCF-7 as examples, 3D multicellular tumor spheroids were prepared and characterized in detail using an inverted microscope, confocal laser microscope, and environmental scanning electron microscope.
Experimental Steps:
1. Preparations Before Experiment
1) Take 12 mL of DMEM (abs9483) and RPMI 1640 (abs9484) complete culture medium (containing 10% FBS (abs972), same below) in advance and place them in a 50 mL centrifuge tube, then put it in the refrigerator at 4℃ for precooling;
2) Take out the prepackaged Matrigel basement membrane matrix (abs9490) from -20℃ and place it in a 4℃ refrigerator 24 hours in advance to allow it to melt into a liquid state;
3) Place sterile 1 mL pipette tips into a sterile 50 mL centrifuge tube and put it in the -20℃ refrigerator for precooling.
2. Agarose-Coated 96-Well Plate
1) Accurately measure 6 mL of RPMI 1640 culture medium (or DMEM culture medium) into two 10 mL glass syringe bottles, add 90 mg of agarose (abs44056213), seal the bottle, and place it in a water bath at 80℃ to heat and dissolve for 30 minutes;
2) After heating, place the syringe bottle in an autoclave and sterilize at 115℃ for 30 minutes;
3) After sterilization, quickly take out the syringe bottle and place it in a laminar flow hood. Pour the agarose solution from the syringe bottle into a sterile sample trough, and use a multichannel pipette to add 60 µL per well into a 96-well plate.
Note: Since agarose solution solidifies at room temperature, it is essential to quickly transfer the agarose solution to the laminar flow hood and add it to the 96-well plate after taking it out of the autoclave.
Additionally, to ensure the agarose does not cool during pipetting, the sample trough and 100 µL pipette tips must also be sterilized simultaneously.
4) After adding the solution, the 96-well plate should be kept level for approximately 30 minutes to allow the agarose in each well to solidify.
3. Preparation of Cell Suspension with Matrigel
1) Take MDA-MB-231 cells (or MCF-7 cells) in the logarithmic growth phase, digest with trypsin (abs47014938), and count the cells. Adjust the cell suspension concentration to 2.0 × 105 cells/mL using RPMI 1640 complete culture medium (or DMEM complete culture medium), and set aside;
2) Spray a beaker filled with crushed ice with alcohol and place it in a laminar flow hood. Take out the RPMI 1640 complete culture medium and the thawed Matrigel from the refrigerator and place them on ice;
Note: Since Matrigel solution solidifies at room temperature, it is essential to maintain a low temperature throughout the operation.
3) Take out the pre-cooled pipette tips and place them in the laminar flow hood. According to the calculated amount (2.5%, v/v), use a pipette to add 300 µL of Matrigel to 12 mL of RPMI 1640 complete culture medium and mix quickly;Note: Since Matrigel solution solidifies at room temperature, the pipette tips used must also be pre-cooled.
4) Add the cell suspension (approximately 600 µL) from step 1 to achieve a cell concentration of 10,000 cells/mL, mix quickly, and set aside for use;
4. Seeding the Cell Suspension into Agarose-Coated 96-Well Plate
1) Place the cell suspension prepared in step 4 above into the sample trough, and use a multichannel pipette to add 200 µL into the agarose-coated 96-well plate;
2) Centrifuge using a microplate centrifuge (abs72035) under the conditions of 4℃, 1000 × g, 10 min.
Note: To maintain sterility when centrifuging the 96-well plate, seal the edges of the plate with parafilm.
3) After centrifugation, take out the 96-well plate, remove the parafilm, spray with alcohol, and place it in the incubator for cultivation. The entire cultivation process is shown in Figure 1.
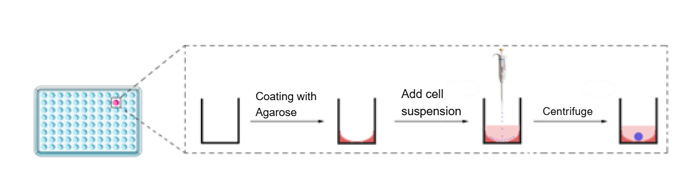
Figure 1
4) On days 3, 5, and 7 of cultivation, replace the 100 µL culture medium in the wells and observe the morphology of the tumor spheroids using an inverted microscope.
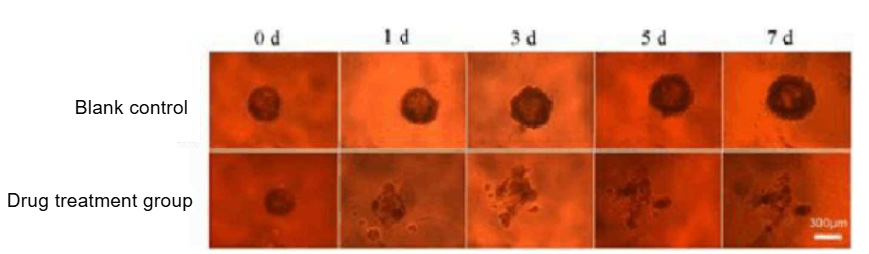
Figure 2
5) If using 3D multicellular tumor spheroids for drug testing, after 7 days of cultivation, use a pipette to remove 100 µL of the culture medium from the wells, add 100 µL of the drug solution, and then place it in the incubator for cultivation. Regularly observe the growth status of the tumor spheroids using an inverted microscope (Figure 2).
5. Characterization of 3D Multicellular Tumor Spheroids
1) Observation of 3D multicellular tumor spheroid morphology using an inverted microscope: Simply place the 96-well plate under the inverted microscope for observation.
2) Observation using a confocal laser microscope: Carefully remove the tumor spheroids from the wells with a pipette, wash three times with PBS, fix with 4% paraformaldehyde (abs9179), and stain the nuclei with Hoechst 33258 (abs47047617). After washing three times with PBS, observe under a confocal laser microscope (Figure 3).

Figure 3
3) Observation using an environmental scanning electron microscope: Carefully remove the tumor spheroids from the wells with a pipette, wash three times with PBS (abs962), fix and dry, and then observe using an environmental scanning electron microscope (Figure 4).
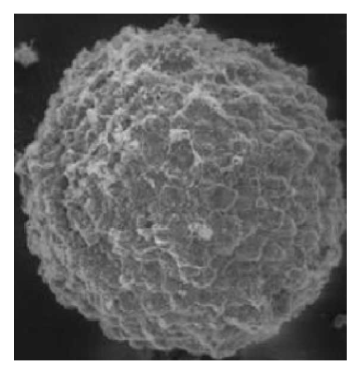
Figure 4
Common Issues:
In practice, we often encounter the issue of cells failing to form spheroids. Below, we explore the reasons for cells not forming spheroids in 3D cell culture and solutions to these problems.
1. Cell Type and Characteristics:
The type and characteristics of cells are one of the key factors affecting their ability to form spheroid structures in a 3D environment. Different types of cells have varying self-organization and adhesion properties, which may be more suitable for forming other types of 3D structures, such as sheets or tubes.
Solution: Before conducting 3D culture experiments, it is essential to carefully select cell types suitable for self-organization. For cells that do not readily form spheroids, consider using inducers or genetic regulation methods to enhance their self-organization capabilities.
2. Lack or Inappropriate Extracellular Matrix (ECM):
The formation of spheroids typically requires appropriate extracellular matrix support. If the ECM components in the culture medium are insufficient or inappropriate, cells may fail to form spheroids.
Solution: Design the ECM components in the culture medium rationally to ensure that cells have sufficient support to form spheroids. Additionally, consider adding exogenous ECM support materials, such as appropriate matrix proteins or bioactive materials.
3. Insufficient Cell Density:
The formation of spheroids may require sufficient cell density to support cell-cell interactions and self-organization. If the cell density is too low, it may affect the formation of spheroid structures.
Solution: Optimize the cell seeding density to ensure an adequate number of cells for the self-organization process.
4. Inappropriate Culture Conditions:
Culture conditions such as medium composition, temperature, and oxygen concentration may affect the cells' ability to form spheroids. Inappropriate culture conditions may hinder the cells' self-organization capabilities.
Solution: Optimize the culture conditions to create an environment more conducive to cell self-organization, such as adjusting the medium composition, temperature, and gas concentration.
II. 3D Tumor Spheroid Matrigel Culture Strategy
1. Preparations Before Experiment
1) Prepare cell culture reagents;
2) Take out the prepackaged Matrigel (abs9495) from -20℃ and place it in a 4℃ refrigerator 24 hours in advance to allow it to melt into a liquid state; place sterile 1 mL pipette tips into a sterile 50 mL centrifuge tube and put it in the -20℃ refrigerator for precooling.
2. Gel Addition and Plate Seeding
1) Take cells in the logarithmic growth phase, digest with trypsin, and count the cells (e.g., for a 24-well plate, seed 7000 cells per well in 25 µL of Matrigel cell suspension). Centrifuge at 300g, 4℃ for 5 minutes, discard the supernatant, and keep the cell pellet for use;
2) Take out the thawed Matrigel and place it on a metal ice bucket (or a beaker filled with crushed ice sprayed with alcohol and placed in a laminar flow hood);
Note: Since Matrigel solution solidifies at room temperature, it is essential to maintain a low temperature during the operation. The pipette tips, EP tubes, centrifuge tubes, and metal ice bucket must be pre-cooled at -20℃.
3) Add Matrigel to the cell pellet (operate on metal ice bucket or ice), mix quickly, and seed the plate (aim to complete within half a minute, as prolonged time will cause Matrigel to aggregate and form bubbles). For example, in a 24-well cell culture plate, add 25 µL of Matrigel mixture per well (in droplet form). Place the seeded plate in the incubator at 37℃ for 10-15 minutes to allow the gel to set, then add 500-750 µL of culture medium for cultivation;
4) On days 3, 5, and 7 of cultivation, replace the culture medium for the spheroids and observe the morphology of the tumor spheroids using an inverted microscope;
5) If using 3D multicellular tumor spheroids for drug testing, after 7 days of cultivation, use a pipette to remove 100 µL of the culture medium from the wells, add 100 µL of the drug solution, and then place it in the incubator for cultivation. Regularly observe the growth status of the tumor spheroids using an inverted microscope.
3. Characterization of 3D Multicellular Tumor Spheroids
1) Observation using an inverted microscope: Simply place the 96-well plate under the microscope for observation;
2) Observation using a confocal laser microscope: Carefully remove the tumor spheroids from the wells with a pipette, wash three times with PBS, fix with 4% paraformaldehyde (abs9179), and stain the nuclei with Hoechst 33258 (abs811667). After washing three times with PBS, observe under a confocal laser microscope;
3) Observation using an environmental scanning electron microscope: Carefully remove the tumor spheroids from the wells with a pipette, wash three times with PBS, fix and dry, and then observe using an environmental scanning electron microscope.
III. 3D Tumor Spheroid Ultra-Low Attachment Culture Strategy
Using breast cancer cells MDA-MB-231 and MCF-7 as examples, 3D multicellular tumor spheroids were prepared and characterized in detail using an inverted microscope, confocal laser microscope, and environmental scanning electron microscope.
1. Preparations Before Experiment
1) Take 12 mL of DMEM (abs9483) and RPMI 1640 (abs9484) complete culture medium (containing 10% FBS (abs972)) in advance and place them in a 50 mL centrifuge tube, then put it in the refrigerator at 4℃ for precooling;
2) Ultra-low attachment culture plate (96-well plate, round bottom) (abs7060).
2. Preparation of Cell Suspension
Take MDA-MB-231 cells (or MCF-7 cells) in the logarithmic growth phase, digest with trypsin (abs47014938), and count the cells. Adjust the cell suspension concentration to 1.43 × 105 cells/mL (i.e., 10,000 cells/70 µL) or other concentrations (i.e., 2500 cells/70 µL, 500 cells/70 µL, 100 cells/70 µL) according to your experimental needs.
3. Seeding the Cell Suspension into Ultra-Low Attachment 96-Well Plate
1) Place the cell suspension prepared in step 2 above into the sample trough, gently mix the cell suspension with a pipette to ensure uniform concentration, and use a multichannel pipette to add the cell suspension into the ultra-low attachment 96-well plate, 70 µL per well.
2) Cover the plate and centrifuge at room temperature, 250 RCF, 2 minutes;
Note: To maintain sterility when centrifuging the 96-well plate, seal the edges of the plate with parafilm.
3) After centrifugation, take out the 96-well plate, remove the parafilm, spray with alcohol, and place it in the incubator for cultivation. Tilt the plate at an angle of 30 degrees to the horizontal plane (as shown in the figure below), which can be achieved by propping up one side of the plate.

Figure 5
4) On days 3, 5, and 7 of cultivation, replace the 100 µL culture medium in the wells and observe the morphology of the tumor spheroids using an inverted microscope;
5) If using 3D multicellular tumor spheroids for drug testing, after 7 days of cultivation, use a pipette to remove 100 µL of the culture medium from the wells, add 100 µL of the drug solution, and then place it in the incubator for cultivation. Regularly observe the growth status of the tumor spheroids using an inverted microscope.
4. Characterization of 3D Multicellular Tumor Spheroids
1) Observation of 3D multicellular tumor spheroid morphology using an inverted microscope: Simply place the 96-well plate under the inverted microscope for observation;
2) Observation using a confocal laser microscope: Carefully remove the tumor spheroids from the wells with a pipette, wash three times with PBS, fix with 4% paraformaldehyde (abs9179), and stain the nuclei with Hoechst 33258 (abs47047617). After washing three times with PBS, observe under a confocal laser microscope;
3) Observation using an environmental scanning electron microscope: Carefully remove the tumor spheroids from the wells with a pipette, wash three times with PBS (abs962), fix and dry, and then observe using an environmental scanning electron microscope.
|
Catalog Number |
Product Name |
Specification |
Application |
|
Matrigel (standard type, with phenol red) |
1.5mL×4 |
Organoid culture; cell invasion; cell migration |
|
|
Matrigel (standard type, phenol red-free) |
1.5mL×8 |
||
|
Matrigel (high concentration, with phenol red) |
1.5mL×8 |
Subcutaneous tumor formation in mice; angiogenesis; gel embolization |
|
|
Matrigel (high concentration, phenol red-free) |
1.5mL×4 |
||
|
Matrigel (low factor, with phenol red) |
1.5mL×4 |
Organoid culture; research related to growth factors, signaling pathways, etc. |
|
|
Matrigel (low factor, phenol red-free) |
1.5mL×4 |
||
|
Matrigel (high concentration, low factor, with phenol red) |
1.5mL×8 |
Subcutaneous tumor formation in mice; angiogenesis; gel embolization; research related to growth factors, signaling pathways, etc. |
|
|
Matrigel (high concentration, low factor, phenol red-free) |
1.5mL×8 |
||
|
Matrigel (IPS validated phenol red-free) |
1.5mL×4 |
Coating the wells of the plate is suitable for the proliferation and maintenance of human embryonic stem cells (hES) and induced pluripotent stem cells (IPS). | |
|
Ready to use Matrigel |
100mL |
The coated plate is suitable for the proliferation and maintenance of adherent-resistant cells (such as 293T and HUVEC). |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
