- Cart 0
- English
Comprehensive Strategies for Matrigel Experiment Applications
March 26, 2025
Clicks:3212
Overview
Matrigel is a basement membrane component extracted from mouse tumor tissue (the basement membrane is a layer of matrix membrane on the basal surface of epithelial cells in animals). The main components of Matrigel are laminin, type IV collagen, and nestin. Additionally, Matrigel contains various growth factors, such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), nerve growth factor (NGF), basic fibroblast growth factor (FGF-2), transforming growth factor-beta (TGF-beta), and insulin-like growth factor (ILGF) (Vukicevic et al. 1992).
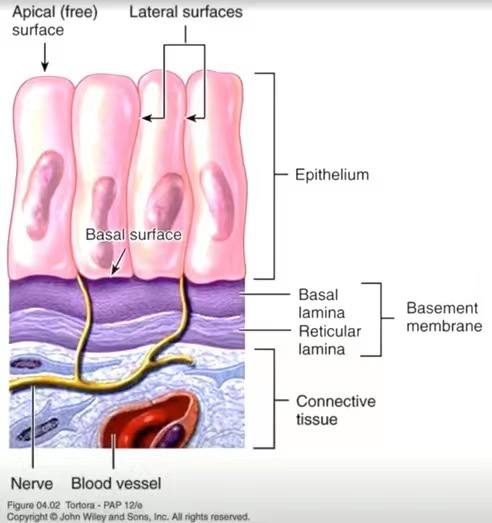
Localization of the basement membrane in mouse tumor tissue
Applications of Matrigel in Various Experimental Fields
1. Mouse Tumor Formation Experiments
Animal experiments are more capable of simulating human physiological and pathological conditions. In tumor research, the most common animal experiment is the nude mouse tumor formation experiment. Since most tumor studies use human cells, and due to the existence of xenogeneic rejection, we need immunodeficient mice as carriers for the xenograft tumor model. By injecting tumor cells into these mice, tumors can be formed to observe the growth of the fluid and judge its biological changes.
Operational Steps
1) Collect 5*106 cells for injection into mice, and mix them with Matrigel at a volume ratio of 1:1. To facilitate injection, it is recommended that the total volume of each injection be no less than 100µL;
2) Depilate the skin at the injection site of the mouse using a depilator, and then disinfect it with an alcohol swab;
3) Use a 1mL syringe without a needle to draw the mixture of cells and gel into the needle, then attach a 16g needle (with an inner diameter of 1.194mm), and inject the mixture subcutaneously or into the thigh muscle (for tumors with particularly high metabolism).
Precautions:
1) Animal selection: Generally, 4-6-week-old Nude mice are chosen, and they need to adapt to the SPF environment for 1 week in advance;
2) Inoculation site and inoculation volume: The common inoculation site is subcutaneous, intravenous, or in situ; the cell volume is generally 5*106 cells/200µL, but there are slight differences in inoculation volumes for different cell lines;
3) Tumor formation time: Cells inoculated subcutaneously usually form tumors within 1-2 weeks, generally not exceeding 1 month; cells inoculated via tail vein and intraperitoneal injection usually take about 1 week, and it is necessary to closely observe the weight and condition of the animals to make a judgment;
4) When injecting, be sure not to leak or inject into the muscle, as this will result in poor consistency of tumor volume. Generally, after entering the needle, the needle tip is lifted upwards, and the injected volume does not exceed 200µL.

Matrigel tumor formation comparison experiment
Using Absin high-concentration Matrigel, the volume of subcutaneous tumors in mice is significantly higher than that of the control group and brand C in the later stage.
2. Organoid Culture
Organoids are three-dimensional (3D) cell cultures that contain key characteristics of their representative organs. This in vitro culture system includes a self-renewing stem cell population that can differentiate into multiple organ-specific cell types, has similar spatial organization to the corresponding organ, and can reproduce some functions of the corresponding organ, thus providing a highly physiologically relevant system. Organoids can be derived from adult stem cells (ASCs), pluripotent stem cells (PSCs) (i.e., embryonic stem cells or ESCs), or induced PSCs (iPSCs). The organoid culture system mainly includes Matrigel, factors required to maintain the organoid ecosystem, and factors required for differentiation. Matrigel contains collagen, nestin, and fibronectin, etc., which provide the matrix for the formation of the three-dimensional spatial structure of organoids. The main purpose of factors maintaining the organoid ecosystem is to promote cell proliferation and inhibit cell apoptosis, etc.
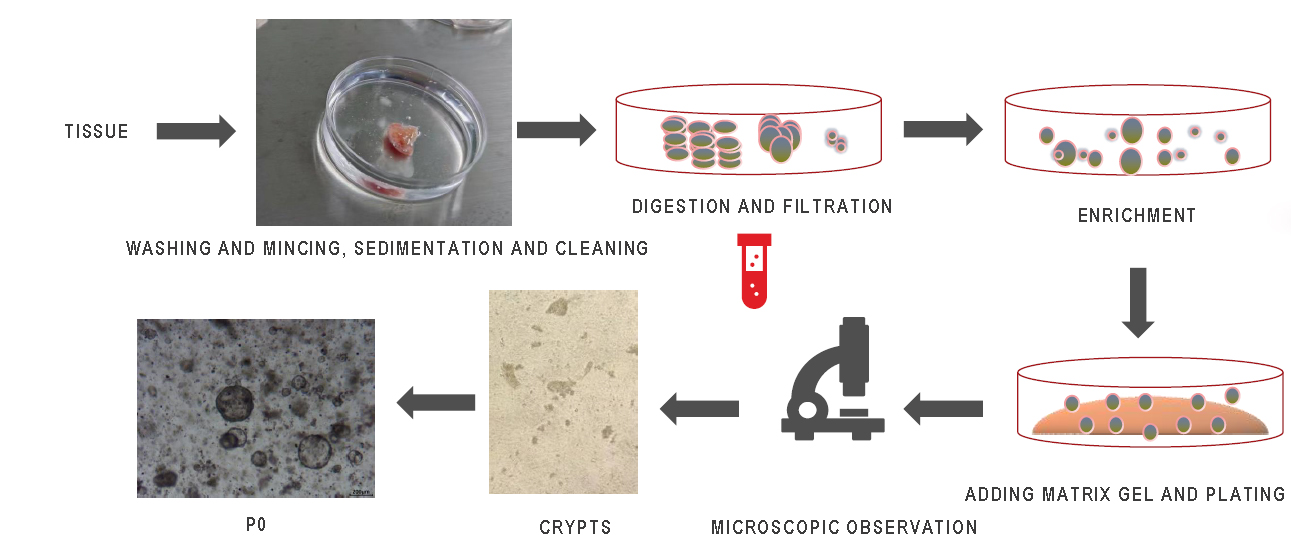
Organoid culture process
Take Human Colorectal Cancer Organoid Culture as an Example
Reagents and Materials
Human Colorectal Cancer Organoid Culture Kit (abs9445), Matrigel (low factor, phenol red-free) (abs9495), 60mm cell culture dish (abs7005), 100μm cell strainer (abs7009), 15mL centrifuge tube (abs7102), several 1.5mL EP tubes (abs7119), 24-well cell culture plate (abs7058), metal ice bucket, ophthalmic scissors, ophthalmic forceps
Kit Components
|
Component Name |
Specification |
|
Human Colorectal Cancer Organoid Culture Medium A |
100mL |
|
Primary Culture Buffer B for Organoids |
250mL |
|
Human Colorectal Cancer Primary Tissue Digestion Liquid C |
30mL |
|
Organoid Passaging Digestion Liquid D |
30mL |
|
Tissue Preservation Liquid E |
100mL |
|
Organoid Cryopreservation Liquid F |
20mL |
|
Organoid Passaging Culture Buffer G |
250mL |
Operational Steps
1) Organoid Operation Process - Sample Preparation
a. Place the tissue into a sampling bottle containing pre-cooled (2-8°C) Tissue Preservation Liquid E (submerge the entire tissue), and transport it from the hospital/laboratory at 4℃;
b. Disinfect the sampling bottle, take out the tissue and place it into a culture dish for sample photography, and register information such as size, color, hardness, and tissue type.
2) Organoid Operation Process - Washing and Cutting
Soak in 2-3mL Primary Culture Buffer B in a 60mm cell culture dish (abs7005). Wash with Primary Culture Buffer B three times (changing the culture dish each time), then cut into approximately 1-3mm3 tissue blocks, and transfer to a 15mL centrifuge tube.
3) Organoid Operation Process - Digestion and Filtration
a. Add Human Colorectal Cancer Primary Tissue Digestion Liquid C to the 15mL centrifuge tube (the digestion liquid is 3-5 times the volume of the tissue) and digest at 37℃ for 10-15min (observe the digestion process at any time);
b. Take a small amount of liquid and observe under a microscope. When observing many cell clusters (5-10 cells per cluster) under the microscope, add 3 times the volume of Primary Culture Buffer B to terminate the digestion. Gently pipette up and down to see the liquid become turbid;
c. Filter using a 100μm cell strainer (abs7009), and take a small amount of the filtrate for microscopic observation. Collect the filtrate into a 15mL centrifuge tube, centrifuge at 300g at 4℃ for 5min to enrich, remove the supernatant, add about 1mL Primary Culture Buffer B to the 1.5mL EP tube, and re-suspend and centrifuge again.
4) Organoid Operation Process - Centrifugation and Gel Addition
Matrigel calculation: After step 3, observe the collected tissue volume, add 25 times the tissue volume of Matrigel (abs9495) to re-suspend and coat the plate (operate on a metal ice bucket or ice).
5) Organoid Operation Process - Gel Dropping and Liquid Addition
Taking a 24-well cell culture plate as an example, spot 25uL of the tissue-Matrigel mixture per well to coat the plate (operate on a metal ice bucket or ice), place the coated culture plate into a 37℃ incubator for 40-60min to gel, and add 500-750μL Human Colorectal Cancer Organoid Culture Medium A for culture.
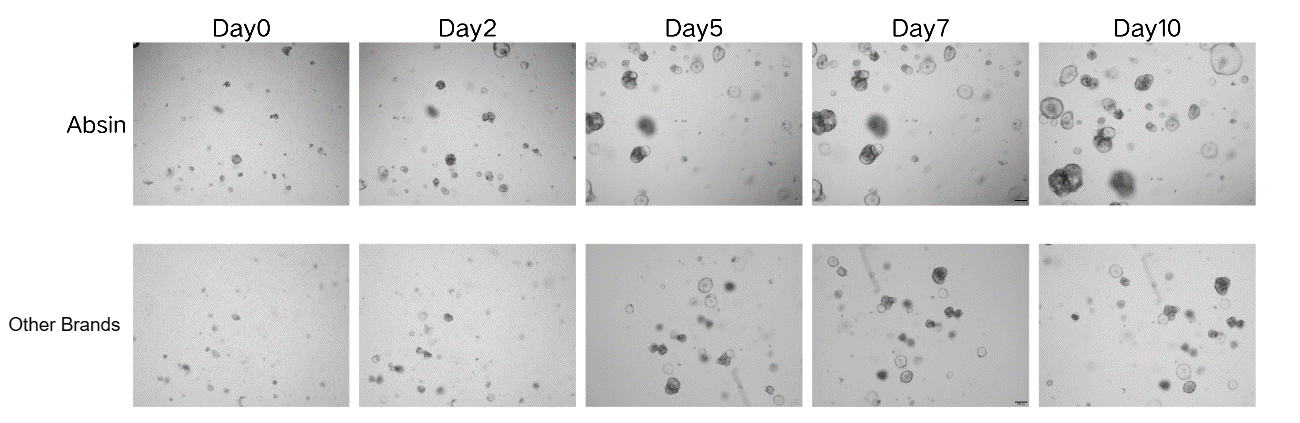
Organoid Culture Comparison
Using Absin low-factor Matrigel, the number and volume of colorectal cancer organoids are significantly greater than those of a certain imported brand.
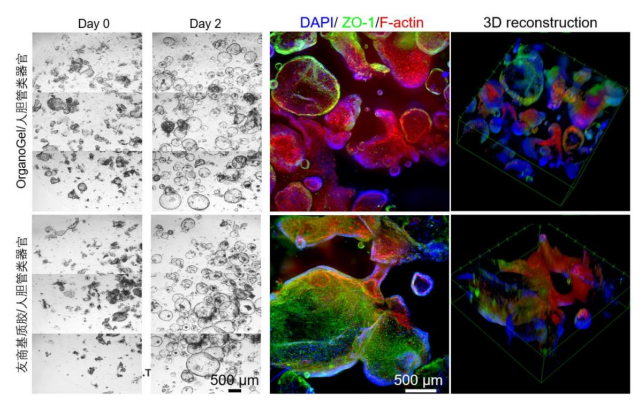
Using Absin low-factor Matrigel, the number of colorectal cancer organoids is significantly greater than that of a friendly brand, and the fluorescence-stained organoids have a more complete morphology. Human biliary tract organoids are imaged by staining with nuclear dye DAPI (blue), tight junction protein antibody anti-ZO1, and F-actin dye Alexa Fluor 647 Phalloidin.
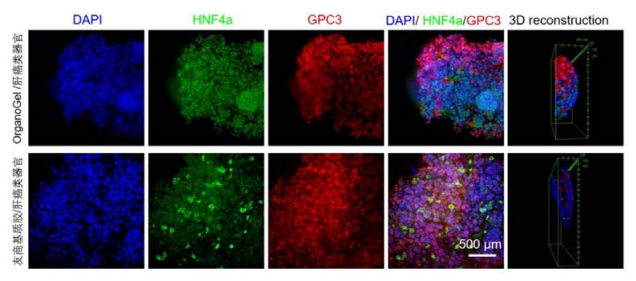
Using Absin low-factor Matrigel, the staining effect of liver cancer organoids is comparable to that of a friendly brand. Liver cancer organoids are imaged by staining with nuclear dye DAPI (blue), liver cell biomarker HNF4a, and liver cancer marker GPC3 antibodies.
3. Angiogenesis Experiment
Angiogenesis refers to the growth of new capillary vessels originating from existing capillaries and post-capillary venules. Angiogenesis is involved in the pathological changes of many diseases. Whether it is primary or secondary tumors, once the growth diameter exceeds 1-2mm, angiogenesis will occur. This is because tumor cells themselves can secrete a variety of growth factors that induce angiogenesis. Controlling angiogenesis is beneficial for the treatment of many diseases. It is of great significance to study the patterns of angiogenesis and regression under disease conditions from a pathological perspective, to adopt effective vascular treatment strategies, and to conduct research on organ metastasis, etc. The tube formation assay is a rapid and quantifiable method for measuring angiogenesis in vitro.
Take the immortalized HUVEC cell line as an example:
Reagents and Materials
Matrigel (standard type, phenol red-free) (abs9491), fetal bovine serum, HUVEC cells (5*104 per well), 96-well cell culture plate (abs7036), penicillin-streptomycin solution (100×, double antibiotics) (abs9244), DMEM high-glucose culture medium (containing L-glutamine, containing sodium pyruvate, without HEPES) (abs9483)
Experimental Steps
1) Replace the complete culture medium with the starvation medium for cells: Add DMEM culture medium containing 0.2% FBS and 1% double antibiotics and culture for 24h;
2) Evenly coat the bottom of the 96-well plate with Matrigel.
Note: The pipette tip needs to be pre-cooled for 30min. Try to operate on ice to avoid premature solidification of Matrigel and the generation of bubbles;
3) Incubate the 96-well plate in a cell culture incubator for 30min to solidify the Matrigel;
4) Digest HUVEC cells and count;
5) Add 200µL of HUVEC cell suspension (containing 5*104 cells) to the 96-well plate containing Matrigel. Place the 96-well plate in the incubator;
6) The vascular-like network structure will form within 3-12h;
7) At the optimal time for vascular network formation, carefully remove the culture medium and stain with culture medium containing live cell dye 1/1000 Calcein AM (green), and take photos with a microscope for record.
4. Cell Invasion
The Transwell cell invasion experiment in vitro is applied to study the effects of various cytokines on the invasion and metastasis of malignant tumor cells and the research of some new drugs that inhibit angiogenesis. The principle of the Transwell experiment is to place the Transwell insert into the culture plate and separate high-nutrient liquid from low-nutrient liquid with a membrane. Cells are placed in the low-nutrient liquid, and in order to obtain more nutrients, cells will pass through this membrane into the high-nutrient liquid. The Transwell cell experiment can be used as a very convenient method to study the migration, invasion, and metastasis of tumor cells.
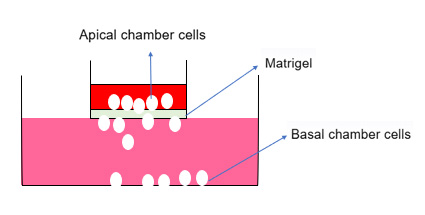
Schematic diagram of cell invasion experiment principle
Experimental Materials
Matrigel (standard type, phenol red-free) (abs9491), fetal bovine serum, PBS (abs962), BSA (abs9156), DMEM high-glucose culture medium (containing L-glutamine, containing sodium pyruvate, without HEPES) (abs9483), 24-well plate cell culture insert with culture plate (abs7282), upper layer culture medium: DMEM with 0.05%-0.2% BSA, lower layer culture medium: DMEM with 5%-10% FBS
Experimental Steps
1) Coat with Matrigel: Under 4℃ conditions, dilute Matrigel with serum-free cell culture medium or PBS buffer at a ratio of 1:8 (the dilution ratio needs to be optimized to select a concentration that allows an appropriate number of cells to pass through). Take 100uL and evenly coat the surface of the polycarbonate membrane in the upper chamber, place at 37℃ for 0.5-1h to polymerize into a gel.
Note:
a. Gently expel the Matrigel along the inner wall of the insert with the pipette tip, being careful not to poke the insert filter membrane;
b. The volume of Matrigel added should not be too large. Just wet the polycarbonate membrane is sufficient;
c. Pay attention to low temperature, and the pipette tip, insert, etc. should all be pre-cooled at 4℃.
2) Cell culture: Take the logarithmic growth phase test cells, wash with PBS, then suspend the cells with upper layer culture medium (DMEM with 0.05%-0.2% BSA), and adjust the cell density to 1-10*105/mL.
3) Cell inoculation: Generally add 500-650uL lower layer culture medium (DMEM with 5%-10% FBS) to the lower chamber of the 24-well plate. Then place the Transwell insert into the 24-well plate with tweezers, take 100-200µL of cell suspension and add it to the upper chamber, and finally place it in the incubator for 12-48h (depending on the metastatic ability of the cancer cells).
Note:
a. Try to avoid the generation of bubbles: Bubbles often occur between the lower layer culture medium and the insert, which will affect the chemotactic effect of the lower layer culture medium. Therefore, if large bubbles appear, lift the insert, remove the bubbles, and then place the insert into the culture plate;
b. Be sure to inoculate the cells evenly, and it is recommended to add slowly along the wall;
c. In addition to considering the metastatic ability of the cells when selecting time points, the impact of treatment factors on the number of cells cannot be ignored, such as some drugs that inhibit cell proliferation.
4) Cell fixation: Take out the insert, aspirate the culture medium, gently wipe the cells on the Matrigel and inside the upper chamber with a cotton swab. Take a new 24-well plate and add 600µL of 4% paraformaldehyde, place the insert in it and fix for 20-30min.
5) Cell staining and counting: Discard the fixation solution, stain with 0.1%-0.2% crystal violet for 5-10min, wash with PBS for 3 times, remove the crystal violet that does not bind to the cells, gently wipe the upper side of the insert with a cotton swab to remove the non-specifically bound dye on the upper surface of the insert for subsequent microscopic examination. After appropriate air-drying, observe the cells in 5 fields of view under a high-power microscope and count.
Recommended number of cells per well for common cell lines (for reference only)
|
Cell Name |
Cell Inoculation Volume |
Detection Time Point |
|
DU145 |
3*104 |
24h |
|
PC3 |
5*104 |
24h |
|
A549 |
8*104 |
48h |
|
22RV1 |
8*104 |
72h |
5. Cell Migration
The cell migration experiment is similar to the cell invasion experiment, with the difference being that the cell migration does not require Matrigel coating.
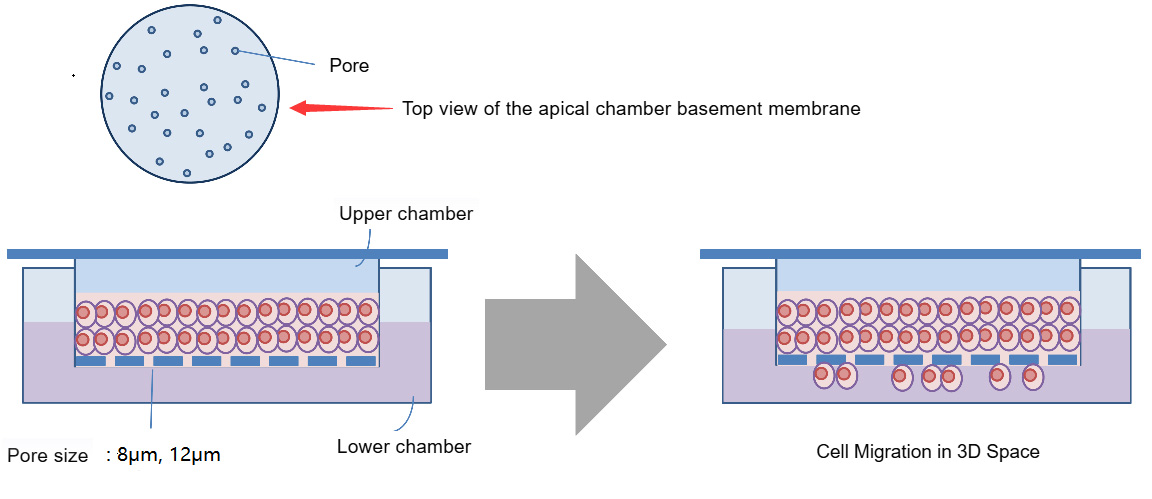
Schematic diagram of cell migration experiment principle
Experimental Reagents
Fetal bovine serum (abs972), DMEM (abs9483), PBS (abs962), BSA (abs9156), 24-well plate cell culture insert with culture plate (abs7282), upper layer culture medium: DMEM with 0.05%-0.2% BSA, lower layer culture medium: DMEM with 5%-10% FBS
Experimental Steps
1) Cell culture: Take the logarithmic growth phase test cells, wash with PBS, then suspend the cells with upper layer culture medium (DMEM with 0.05%-0.2% BSA), and adjust the cell density to 1-10*105/mL.
2) Cell inoculation: Generally add 500-650uL lower layer culture medium (DMEM with 5%-10% FBS) to the lower chamber of the 24-well plate (some special experiments can add chemotactic factors). Then place the Transwell insert into the 24-well plate with tweezers, take 100-200µL of cell suspension and add it to the upper chamber, and finally place it in the incubator for 12-48h (depending on the metastatic ability of the cancer cells).
Note:
a. Try to avoid the generation of bubbles: Bubbles often occur between the lower layer culture medium and the insert, which will affect the chemotactic effect of the lower layer culture medium. Therefore, if large bubbles appear, lift the insert, remove the bubbles, and then place the insert into the culture plate;
b. Be sure to inoculate the cells evenly, and it is recommended to add slowly along the wall;
c. In addition to considering the metastatic ability of the cells when selecting time points, the impact of treatment factors on the number of cells cannot be ignored, such as some drugs that inhibit cell proliferation.
3) Cell fixation: Take out the insert, aspirate the culture medium, gently wipe the cells inside the upper chamber with a cotton swab. Take a new 24-well plate and add 600µL of 4% paraformaldehyde, place the insert in it and fix for 20-30min.
4) Cell staining and counting: Discard the fixation solution, stain with 0.1%-0.2% crystal violet for 5-10min, wash with PBS for 3 times, remove the crystal violet that does not bind to the cells, gently wipe the upper side of the insert with a cotton swab to remove the non-specifically bound dye on the upper surface of the insert for subsequent microscopic examination. After appropriate air-drying, observe the cells in 5 fields of view under a high-power microscope and count.
*Note: Some of the images in this article are sourced from the Internet for learning and reference purposes only.
|
Catalog Number |
Product Name |
Specification |
Application |
|
Matrigel (standard type, with phenol red) |
1.5mL×4 |
Organoid culture; cell invasion; cell migration |
|
|
Matrigel (standard type, phenol red-free) |
1.5mL×8 |
||
|
Matrigel (high concentration, with phenol red) |
1.5mL×8 |
Subcutaneous tumor formation in mice; angiogenesis; gel embolization |
|
|
Matrigel (high concentration, phenol red-free) |
1.5mL×4 |
||
|
Matrigel (low factor, with phenol red) |
1.5mL×4 |
Organoid culture; research related to growth factors, signaling pathways, etc. |
|
|
Matrigel (low factor, phenol red-free) |
1.5mL×4 |
||
|
Matrigel (high concentration, low factor, with phenol red) |
1.5mL×8 |
Subcutaneous tumor formation in mice; angiogenesis; gel embolization; research related to growth factors, signaling pathways, etc. |
|
|
Matrigel (high concentration, low factor, phenol red-free) |
1.5mL×8 |
||
|
Matrigel (IPS validated phenol red-free) |
1.5mL×4 |
Coating the wells of the plate is suitable for the proliferation and maintenance of human embryonic stem cells (hES) and induced pluripotent stem cells (IPS). | |
|
Ready to use Matrigel |
100mL |
The coated plate is suitable for the proliferation and maintenance of adherent-resistant cells (such as 293T and HUVEC). |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
