- Cart 0
- English
Ultimate Tips and Techniques for Organoid Culture
Overview
With the increasing demand for organoid models in experiments, organoid culture has become an essential experimental skill. However, organoid culture is relatively complex compared to cell culture, and many details need to be paid attention to. A detailed protocol is required to guide us in quickly mastering organoid culture techniques. The culture steps for different types of organoids are similar, so once we have mastered the general method, we can make appropriate adjustments for different organoids. Today, we present the most comprehensive guide to organoid culture ever, and you will regret not reading it for ten thousand years!
Procedure
There are various methods for organoid culture, such as using ultra-low attachment culture plates and bioreactors, and the matrix gel method, among others. Today, we will focus on the matrix gel method.

Primary Organoid Culture Workflow
Primary Culture (Taking Human Colorectal Cancer Organoids as an Example)
I. Preparation
1. Equipment
CO2 incubator, double-person single-sided laminar flow hood, inverted microscope, benchtop refrigerated centrifuge, water bath (abs72023) or water bath shaker, medical refrigerator, -80℃ freezer, pipette set, ophthalmic scissors, ophthalmic forceps.
2. Reagents and Consumables (Taking Colorectal Cancer as an Example)
Human Colorectal Cancer Organoid Culture Medium Kit (abs9445), Matrix Gel (Low Factor, Phenol Red Free) (abs9495), 60mm cell culture dish (abs7005), 100μm cell strainer (abs7009), 15mL centrifuge tube (abs7102), several 1.5mL EP tubes (abs7119), 24-well cell culture plate (abs7058), metal ice bucket, ophthalmic scissors, ophthalmic forceps.
|
Component Name |
Specification |
|
Human Colorectal Cancer Organoid Culture Medium A |
100mL |
|
Primary Culture Buffer B |
250mL |
|
Human Colorectal Cancer Tissue Digestion Solution C |
30mL |
|
Organoid Passaging Digestion Solution D |
30mL |
|
Tissue Preservation Solution E |
100mL |
|
Organoid Cryopreservation Solution F |
20mL |
|
Passaging Buffer G |
250mL |
II. Operating Procedure
1. Sample Preparation
(1) Place the tissue into a sampling bottle containing pre-cooled (2-8°C) Tissue Preservation Solution E (submerge the entire tissue), and transport it back from the hospital/laboratory at 4℃.
(2) Sterilize the sampling bottle, remove the tissue and place it into a culture dish for sample photography, and register information such as size, color, texture, and tissue type.

2. Washing and Cutting
(1) Immerse the tissue in a 60mm cell culture dish (abs7005) with 2-3mL of Primary Culture Buffer B. Wash the tissue three times with Primary Culture Buffer B (changing the dish each time) and then cut it into approximately 1-3mm3 tissue pieces, and transfer to a 15mL centrifuge tube.
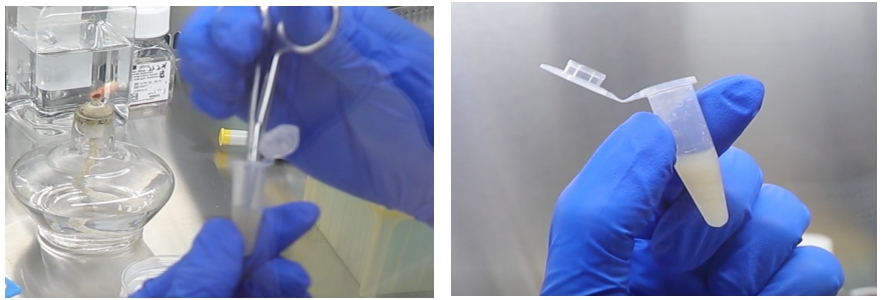
3. Digestion and Filtration
(2) Add 5 times the volume of Human Colorectal Cancer Primary Tissue Digestion Solution C to the 15mL centrifuge tube (digestion solution volume: tissue volume = 5:1, if it is difficult to estimate the tissue volume, 5mL of digestion solution is usually sufficient) and digest at 37℃ for 15-30min (monitor the digestion process at any time).
(3) Take a small amount of liquid and observe under a microscope. When many cell clusters (5-50 cells per cluster) are observed, add 3 times the volume of Primary Culture Buffer B (buffer volume: digestion solution volume = 3:1) to terminate the digestion. Gently pipette the mixture to see the liquid become turbid.
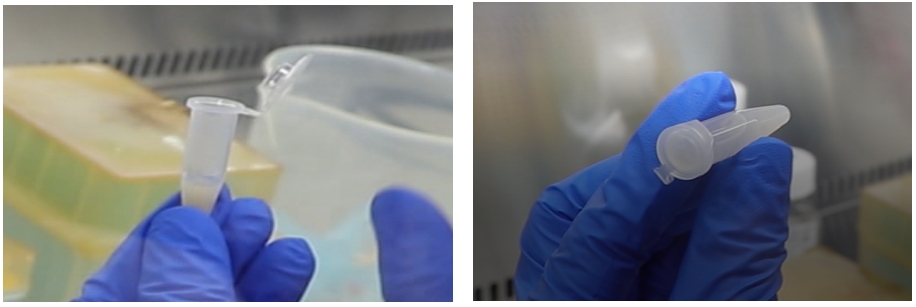
(4) Filter using a 100μm cell strainer (abs7009) and observe a small amount of the filtrate under the microscope. Collect the filtrate into a 15mL centrifuge tube, centrifuge at 300g at 4℃ for 5min, and then remove the supernatant.

4. Gel Addition - Plate Seeding - Medium Addition (The Key Step of Primary Culture)
(1) Preparation
a. The matrix gel should be thawed overnight in a metal ice bucket placed in a 4℃ refrigerator.
b. Pipette tips and centrifuge tubes need to be pre-chilled at -20℃ for at least half an hour.
c. The thawed matrix gel can be stored at 4℃, and it is recommended to use it within 2 weeks.

Low-Temperature Metal Ice Bucket (abs7289)
(2) Seeding Requirements
24-well plate (abs7035), 25uL matrix gel-cell cluster mixture per well, 500-750uL organoid culture medium
(3) Seeding Density
Density Suggestion 1: Matrix gel volume: Cell cluster sediment volume = 25:1 (if it is difficult to estimate the cell cluster sediment volume, 300uL of matrix gel is usually sufficient)
Density Suggestion 2: 500 cell clusters/25uL matrix gel (if counting for seeding is desired, this density suggestion can be referred to)
(4) Gel Addition - Plate Seeding
Add the matrix gel (abs9495) to the cell cluster sediment, gently pipette to mix (do not fully pipette to avoid bubble formation), and then seed the plate. The entire operation should be conducted on a metal ice bucket or ice. Once proficient, the gel addition, mixing, and seeding should be completed within half a minute to maintain the good fluidity of the matrix gel.
(5) Medium Addition
Place the seeded culture plate in a 37℃ incubator for 40-60min to allow the gel to set, then add 500-750μL of Human Colorectal Cancer Organoid Culture Medium A for cultivation. After approximately 10-14 days, when the majority of organoids have diameters of 200um-500um, they can be passaged.
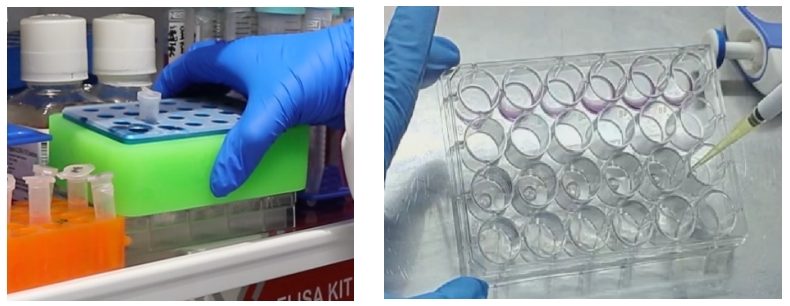
II. When the Number of Organoids is Insufficient or the Size is Small:
1. Collection, Blowing, and Washing of Organoids
(1) Use a pipette to remove the culture medium, and add about 1-2mL of Organoid Passaging Culture Buffer G at 4℃ to each well, gently blowing to disperse the matrix gel.
(2) Blow to form cell clusters from the organoids (stop blowing when many cell clusters are observed under the microscope);
(3) Washing: For a 24-well plate, collect every 5 wells into a 15mL centrifuge tube, and add Organoid Passaging Culture Buffer G to a final volume of 14mL (the more buffer used, the more diluted the matrix gel becomes, making it easier to remove), then place at 4℃ for 40min or -20℃ for 5min (the purpose is to soften the matrix gel; if the refrigerator has strong insulation, shorten the freezing time. When exploring the appropriate freezing time, shake the centrifuge tube and if no matrix gel is visible, it indicates that the cold treatment is complete).
(4) Next, centrifuge the centrifuge tube at 300g, 4℃ for 5min. Typically, two scenarios will occur after centrifugation:
The first scenario is normal, resulting in three layers (as shown in the figure below). In this case, discard the supernatant and matrix gel layer, and retain the cell cluster sediment.

The second scenario is abnormal, resulting in two layers (as shown in the figure below). This situation may be related to insufficient cold treatment. In this case, discard the buffer solution and retain the matrix gel-organoid suspension. Repeat the washing step and re-centrifuge after cold treatment, which usually results in clear stratification (buffer layer, matrix gel layer, organoid sediment layer). At this point, discard the supernatant and matrix gel layer, and retain the organoid sediment. If it still results in two layers (buffer layer and matrix gel-organoid suspension layer), discard the buffer solution and the top 1/3 of the matrix gel-organoid suspension, and retain the bottom 2/3.

Conditions to Promote Effective Separation of Matrix Gel and Organoids:
a. The choice of centrifuge is very important; a horizontal angle centrifuge is more effective for separating matrix gel and organoids compared to a fixed-angle centrifuge;
b. The optimal temperature for the centrifuge is 4℃ (to prevent matrix gel solidification), the centrifugation speed can be moderately increased (not exceeding 500g), and the centrifugation time can be extended (not exceeding 10min).
2. Gel Addition - Plate Seeding - Medium Addition
(1) Preparation
(1) The matrix gel should be thawed overnight in a metal ice bucket placed in a 4℃ refrigerator.
(2) Pipette tips and centrifuge tubes need to be pre-chilled at -20℃ for at least half an hour.
(3) The thawed matrix gel can be stored at 4℃, and it is recommended to use it within 2 weeks.
(2) Seeding Requirements
24-well plate (abs7035), 25uL matrix gel-organoid mixture per well, 500-750uL organoid culture medium
(3) Seeding Density
Density Suggestion 1: For cases with a smaller number of organoids, to maintain the paracrine signals required for growth, 2-3 wells should be concentrated into 1 well. For example, if 6 wells are collected from a 24-well plate, 2 wells are concentrated into 1 well, and 3 wells are seeded, the amount of matrix gel needed is25*3=75uL.
Density Suggestion 2: 500 cell clusters/25uL matrix gel (if counting for seeding is desired, this density suggestion can be referred to)
Note: Whether following Density Suggestion 1 or 2, if there is residual matrix gel, the new gel volume should be at least 1.5 times the residual gel volume.
(4) Gel Addition - Plate Seeding
Add the matrix gel (abs9495) to the organoid sediment, gently pipette to mix (do not fully pipette to avoid bubble formation), and then seed the plate. The entire operation should be conducted on a metal ice bucket or ice. Once proficient, the gel addition, mixing, and seeding should be completed within half a minute to maintain the good fluidity of the matrix gel.
(5) Medium Addition
Place the seeded culture plate in a 37℃ incubator for 40-60min to allow the gel to set, then add 500-750μL of Human Colorectal Cancer Organoid Culture Medium A for cultivation. After approximately 7-10 days, when the majority of organoids have diameters of 200-300um, they can be passaged again.
Cryopreservation
Key Points for Cryopreservation:
Organoids do not require digestion (the viability of organoids after digestion is low);
Cryopreserve during the exponential growth phase of organoids (the activity of organoids is lower at the time of passaging compared to the exponential phase), which is typically on Day 3-Day 4 after passaging, when the majority of organoids have diameters of 100um-200um. This is the optimal time for cryopreservation.
I. Collection and Washing of Organoids
1. Collection: Use a pipette to remove the culture medium, and add about 1-2mL of Organoid Passaging Culture Buffer G at 4℃ to each well, gently blowing to disperse the matrix gel, and collect into a 15mL centrifuge tube (for a 24-well plate, collect every 5 wells as a group).
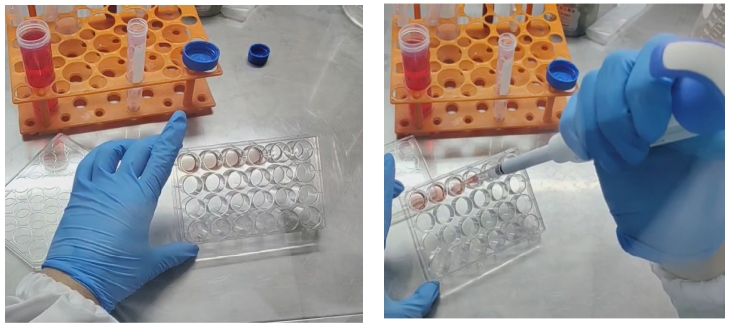
2. Washing: Add Organoid Passaging Culture Buffer G to a final volume of 14mL (the more buffer used, the more diluted the matrix gel becomes, making it easier to remove), then place at 4℃ for 40min or -20℃ for 5min (the purpose is to soften the matrix gel; if the refrigerator has strong insulation, shorten the freezing time. When exploring the appropriate freezing time, shake the centrifuge tube and if no matrix gel is visible, it indicates that the cold treatment is complete).
3. Next, centrifuge the centrifuge tube at 300g, 4℃ for 5min. Typically, two scenarios will occur after centrifugation:
The first scenario is normal, resulting in three layers (as shown in the figure below). In this case, discard the supernatant and matrix gel layer, and retain the organoid sediment.

The second scenario is abnormal, resulting in two layers (as shown in the figure below). This situation may be related to insufficient cold treatment. In this case, discard the buffer solution and retain the matrix gel-organoid suspension. Repeat the washing step and re-centrifuge after cold treatment, which usually results in clear stratification (buffer layer, matrix gel layer, organoid sediment layer). At this point, discard the supernatant and matrix gel layer, and retain the organoid sediment. If it still results in two layers (buffer layer and matrix gel-organoid suspension layer), discard the buffer solution and the top 1/3 of the matrix gel-organoid suspension, and retain the bottom 2/3.

Conditions to Promote Effective Separation of Matrix Gel and Organoids:
a. The choice of centrifuge is very important; a horizontal angle centrifuge is more effective for separating matrix gel and organoids compared to a fixed-angle centrifuge;
b. The optimal temperature for the centrifuge is 4℃ (to prevent matrix gel solidification), the centrifugation speed can be moderately increased (not exceeding 500g), and the centrifugation time can be extended (not exceeding 10min).
II. Cryopreservation of Organoids
1. Cryopreservation Density, Taking a 24-well Plate as an Example
Density Suggestion1:2 wells/mL cryopreservation solution
Density Suggestion2:500 organoids/mL cryopreservation solution (if counting for cryopreservation is desired, this density suggestion can be referred to)
2. Add an appropriate amount of Organoid Cryopreservation Solution F, gently pipette to resuspend, and it is recommended to proceed with cryopreservation immediately. (Leaving it for too long can cause damage to organoids due to DMSO)
3. Gradient Cryopreservation: Place the cryovial in a gradient cryopreservation box and then store it at -80℃ overnight. On the second day, transfer it to a liquid nitrogen tank.
Manual Cryopreservation: Place in a 4℃ refrigerator for 30min, transfer to -20℃ for 1h, then move to -80℃ overnight, and transfer to a liquid nitrogen tank on the second day.
Revival
I. Preparations Before Experiment
1. Preheat the water bath to 37℃;
2. Perform routine disinfection in the cell laboratory, spray with preventive spray, and irradiate the surface of the laminar flow hood with ultraviolet light for 40min;
3. Arrange the sterilized centrifuge tubes, pipettes, culture plates, etc. in order in the laminar flow hood.
II. Retrieval of Cryovial
1. Locate the required organoid number according to the cryopreservation record and label.
2. Retrieve the cryovial from the liquid nitrogen tank, and check the number on the cryovial.
III. Rapid Thawing
1. Quickly immerse the cryovial into the preheated water bath for thawing, and continuously shake it to rapidly dissolve the liquid inside.
2. After approximately 1-2min, when the liquid inside the cryovial is completely dissolved, remove it, wipe the outer wall of the cryovial with an alcohol swab, and then transfer it into the laminar flow hood.
IV. Transfer the organoid cryopreservation solution to a 15mL centrifuge tube, add 10 times the volume of Organoid Passaging Culture Buffer G (buffer volume: cryopreservation solution volume = 10:1) to resuspend, gently pipette to mix, centrifuge at 300g, 4℃ for 5min, and discard the supernatant.
V. Gel Addition - Plate Seeding - Medium Addition
1. Preparations
(1) The matrix gel should be thawed overnight in a metal ice bucket placed in a 4℃ refrigerator.
(2) Pipette tips and centrifuge tubes need to be pre-chilled at -20℃ for at least half an hour.
(3) The thawed matrix gel can be stored at 4℃, and it is recommended to use it within 2 weeks.
2. Revival Requirements
24-well plate (abs7035), 25uL matrix gel-organoid mixture per well, 500-750uL organoid culture medium
3. Revival Density
Revival Density Suggestion 1: 1:1 seeding (revive the same number of wells as originally frozen)
Revival Density Suggestion 2: 250 organoids/25uL matrix gel (if counting for seeding is desired, this density suggestion can be referred to)
4. Gel Addition - Plate Seeding
Add the matrix gel (abs9495) to the organoid sediment, gently pipette to mix (do not fully pipette to avoid bubble formation), and then seed the plate. The entire operation should be conducted on a metal ice bucket or ice. Once proficient, the gel addition, mixing, and seeding should be completed within half a minute to maintain the good fluidity of the matrix gel.
5. Medium Addition
Place the seeded culture plate in a 37℃ incubator for 40-60min to allow the gel to set, then add 500-750μL of Human Colorectal Cancer Organoid Culture Medium A for cultivation. After approximately 10-14 days, when the majority of organoids have diameters of 200um-500um, they can be passaged.
Recommended Organoid Culture Products
|
Catalog Number |
Product Name |
Specification |
|
Matrix Gel (Low Factor, Phenol Red Free) |
1.5mL×4 |
|
|
Organotial Human Bladder Cancer Organoid Culture Kit |
1kit |
|
|
Organotial Human Esophageal Cancer Organoid Culture Kit |
1kit |
|
|
Organotial Human Cervical Cancer Organoid Culture Kit |
1kit |
|
|
Organotial Human Glioma Organoid Culture Kit |
1kit |
|
|
Organotial Human Nasopharyngeal Carcinoma Organoid Culture Kit |
1kit |
|
|
Organotial Human Prostate Cancer Organoid Culture Kit |
1kit |
|
|
Organotial Human Laryngeal Carcinoma Organoid Culture Kit |
1kit |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
March 24, 2025
Clicks:333
