- Cart 0
- English
Why Your Phosphorylated Protein Western Blot Isn't Working (And How to Fix It)
March 06, 2025
Clicks:2129
Western Blot is a required skill for researchers, and many have become proficient in it. However, when it comes to phosphorylated protein Western Blots, even seasoned researchers may feel perplexed. Let's delve into the intricacies of phosphorylated protein Western Blots!
Protein Phosphorylation:
Protein phosphorylation refers to the process where, under the catalysis of kinases, the phosphate group from ATP is transferred to the side chains of amino acids in proteins. This process is generally reversible and can be removed by phosphatases. Phosphorylation typically occurs on serine, threonine, and tyrosine residues. Protein phosphorylation can alter protein structure and activity, thereby influencing cellular metabolism, proliferation, differentiation, and other physiological processes. It is the most fundamental, widespread, and crucial mechanism for regulating and controlling protein activity and function. Current research areas include disease mechanisms, cell signaling pathways, drug development, developmental biology, and stress responses.
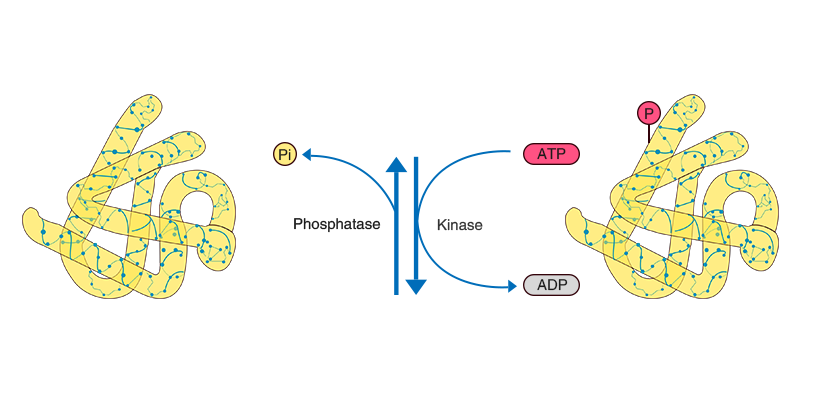
Schematic diagram of protein phosphorylation
Why is Western Blotting of Phosphorylated Proteins Challenging?
1) Phosphorylated proteins are usually present in low abundance, accounting for approximately 10% or even less of the total protein content;
2) Protein phosphorylation is reversible. Once cells are lysed, proteases and phosphatases are immediately released, leading to rapid dephosphorylation of proteins within milliseconds.
Phosphorylated Protein Western Blot: Proceed with Caution
1. Pre-Experiment Preparation—Understand Your Target Protein
It is essential to review literature or consult databases to identify phosphorylation sites and levels of the target protein. We recommend using PhosphoSitePlus—a comprehensive database for post-translational modifications. By entering the protein name, you can query various modification types, phosphorylation sites, and additional data. For proteins with low expression levels, physical or chemical stimulation and induction can be applied.
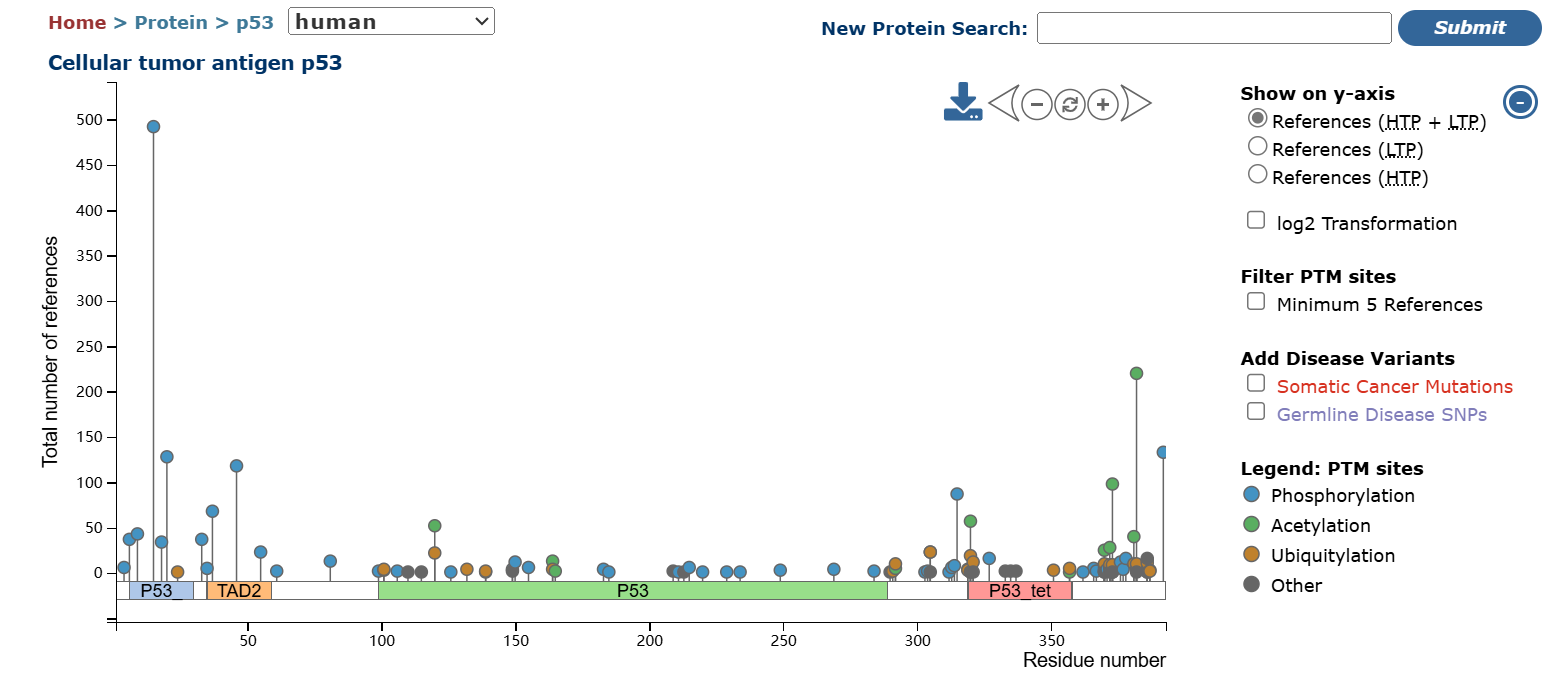

PhosphoSitePlus Database
2. Sample Processing—The First Crucial Step
1) Use fresh samples;
2) Perform operations swiftly;
3) Pre-chill reagents and equipment, and conduct operations on ice;
4) Avoid using ultrasonic cell disruptors if possible. Pre-chill the mortar with liquid nitrogen for tissue grinding;
5) Ensure the lysis buffer contains both protease and phosphatase inhibitors;
6) Avoid boiling some phosphorylated proteins, as this may disrupt their phosphorylation sites;
7) After measuring the concentration, add loading buffer, aliquot, and store at -80°C to prevent repeated freeze-thaw cycles.
3. Internal Controls
Two internal controls are necessary: one for the total protein corresponding to the phosphorylated protein, as changes in phosphorylated protein expression alone are insufficient. Only changes in the ratio of phosphorylated protein to total protein indicate alterations in phosphorylation levels. The other is a common internal control for the experimental system. However, loading and comparing samples in separate lanes can introduce significant errors. A common method is to use antibody stripping solution to remove the primary and secondary antibodies for phosphorylated proteins, then re-probe the same membrane for total protein. This process can be repeated for loading controls.

Expression of phosphorylated CREB1 in the PFC of model rats
4. Loading—Ensure Sufficient Quantity
Compared to conventional Western Blotting, phosphorylated proteins are present in lower levels, so sufficient loading is crucial.
5. Blocking—Choose Wisely
Milk contains a phosphoprotein, casein, which can cause high background signals. For phosphorylated protein detection, it is recommended to use Bovine Serum Albumin (BSA) or a protein-free blocker. Of course, if the antibody instructions suggest otherwise, follow those guidelines.
6. Antibody Incubation
For phosphorylated protein primary antibodies, overnight incubation at 4°C is optimal to ensure sufficient binding time. At 4°C, the antigen is less likely to detach from the membrane. Secondary antibodies can be incubated at room temperature for 1 hour.
7. Washing—Attention to Detail Matters
For initial experiments, reduce the number of washes and the shaker speed. It is better to have some background than no signal at all. Optimization can be done later to achieve ideal results.
References
Pan B, Zhu X, Han B, Weng J, Wang Y, Liu Y. The SIK1/CRTC2/CREB1 and TWIST1/PI3K/Akt/GSK3β signaling pathways mediated by microRNA-25-3p are altered in the schizophrenic rat brain. Front Cell Neurosci. 2023 Jan 20;17:1087335. doi: 10.3389/fncel.2023.1087335. PMID: 36744005; PMCID: PMC9896578.
Absin Phosphorylated Antibodies
|
Catalog Number |
Product Name |
Specification |
|
Rabbit anti-Phospho-NF-kappaB p65(Ser536) Polyclonal Antibody |
100ug |
|
|
Rabbit anti-Phospho-ASK1(Thr845) Polyclonal Antibody |
100uL | |
|
Rabbit anti-Phospho-β Catenin(Tyr333) Polyclonal Antibody |
100uL | |
|
Rabbit anti-Phospho-CREB-1(Ser133) Polyclonal Antibody |
100uL | |
|
Rabbit anti-Phospho-CXCR2(Ser347) Polyclonal Antibody |
100uL | |
|
Rabbit anti-Phospho-E2F1(S337) Polyclonal Antibody |
50uL | |
|
Rabbit anti-Phospho-EphA2(S897) Polyclonal Antibody |
50uL | |
|
Rabbit anti-Phospho-ERK1/2(Thr202+Tyr204) Polyclonal Antibody |
100uL |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
