- Cart 0
- English
Solutions for ADC Drug Development
March 04, 2025
Clicks:595
I. Introduction to Antibody-Drug Conjugates (ADCs)
Antibody-Drug Conjugates (ADCs) are a novel class of highly efficient biotherapeutics that link monoclonal antibodies to cytotoxic small molecules via a chemical linker (Figure 1).
The three key components of ADCs include:
1) Target-specific antibodies;
2) Cytotoxic payloads;
3) Cleavable or non-cleavable linkers.
The specific targeting and effective cytotoxicity of ADCs make them the "biological missiles" in cancer therapy. ADC development involves multiple technical challenges, including rational target selection, optimal combination of antibodies, linkers, and cytotoxic drugs, conjugation technology, and addressing ADC resistance. Target selection, linker choice, conjugation methods, and preclinical efficacy evaluation are the focal points in ADC research and development.

Figure 1 Structure and characteristics of ADCs[1]
II. Preparation of ADCs
The preparation of ADCs begins with the selection of the target antigen, and the safety and efficacy of ADCs largely depend on the choice of the target antigen and its interaction. Highly specific antibodies against the target antigen are then developed, followed by conjugation of the cytotoxic payload to the antibody via lysine, cysteine, or site-specific conjugation methods using cleavable or non-cleavable linkers.
Two common approaches for ADC preparation are one-step and two-step methods. The one-step method involves linking the cytotoxic payload to the linker first, followed by antibody conjugation. The two-step method involves linking the linker to the antibody first, followed by payload conjugation. One of the biggest challenges in ADC development is selecting the appropriate linker and conjugation mode for the antibody and cytotoxic payload. In addition to effectively delivering the cytotoxic payload, the stability of the antibody-payload conjugation system is a key factor determining ADC efficacy and toxicity. It is crucial to ensure that the ADC remains stable in circulation before reaching tumor cells to prevent off-target effects, while efficiently releasing the cytotoxic payload upon reaching tumor cells. The cytotoxic payload is the core component of ADCs, responsible for killing tumor cells either directly or through bystander effects. High potency of the payload is essential for achieving the desired therapeutic effect.
III. Key Elements of ADCs[2]
ADCs consist of antibodies, linkers, and cytotoxic small molecules. The specificity of the target antigen and the conjugation method are crucial for ensuring the safety and efficacy of ADCs in clinical applications.
1. Starting Point of ADC Design: Antigen Targets
The selection of antigen targets should consider the following aspects:
1) High expression of the target antigen in tumors and low or no expression in normal cells;
2) Localization of the target antigen on the cell membrane surface for recognition by specific antibodies;
3) Resistance to shedding to prevent binding of antibodies to circulating antigens;
4) Internalization properties of the target antigen to facilitate the delivery of ADC-linked toxins into tumor cells, without downregulation following ADC treatment.
Over 50 antigens have been identified as targets for ADCs. Solid tumor-associated antigens include HER2, EGFR, CD56, Trop2, CD70, Tissue factor, and Collagen IV. Commonly targeted antigens in ADCs include HER2, CD19, CD33, CD22, and MSLN (mesothelin).
2. Antibodies in ADCs
Antibodies are another critical component of ADCs, which must specifically recognize the target antigen with high affinity. Lack of specificity or cross-reactivity can lead to unpredictable side effects. Antibodies should also exhibit low immunogenicity, long half-life, and stability in circulation. Antibodies are classified into five classes based on their heavy-chain constant region sequences: IgG, IgA, IgD, IgE, and IgM. All approved ADCs utilize IgG antibodies, with IgG1 being the most commonly used due to its moderate molecular weight, high affinity, long half-life, ease of production, and strong Fc effector functions.
ADCs commonly employ chimeric antibodies (mouse variable regions with human constant regions, e.g., Adcetris) and humanized antibodies (mouse CDRs with human sequences, e.g., Mylotarg and Kadcyla) to reduce immunogenicity and minimize the production of human anti-mouse antibodies. However, early ADCs still faced challenges such as off-target toxicity, heterogeneous products, aggregation, rapid clearance, and narrow therapeutic windows.
The specificity, affinity, and internalization rate of ADC antibodies are also important considerations. High specificity ensures that cytotoxic molecules are concentrated at tumor sites, achieving targeted pharmacological effects. Low specificity increases the risk of toxicity to normal tissues. ADC antibodies should exhibit high binding affinity, typically in the range of 0.1-1.0 nM. Compared to small molecules, antibodies enter tissues more slowly from the plasma, and faster internalization rates can enhance ADC efficacy.
ADC antibodies can also be in the form of bispecific antibodies or single-domain antibodies. Bispecific antibodies can target two different epitopes of the same antigen or two different antigens. Some ADCs use single-domain antibodies conjugated to cytotoxic molecules or radioactive elements to develop novel ADCs.
3. Linkers in ADCs
The linker connects the antibody to the cytotoxic payload. Stability of the linker in circulation is crucial to maintain the attachment of the cytotoxic payload to the antibody. However, once ADCs enter tumor cells or lysosomes, the linker should rapidly degrade to release the payload. Linkers influence many important properties of ADCs, such as drug-to-antibody ratio (DAR), payload release kinetics, therapeutic index (TI), and pharmacokinetics.
Based on their cleavage mechanisms within tumor cells, linkers can be classified as cleavable or non-cleavable. Cleavable linkers can be released through various mechanisms, including acid-labile hydrazone bonds, reducible disulfide bonds, and enzymatically cleavable peptide bonds. For example, acid-labile linkers are stable in circulation but rapidly cleave in the acidic lysosomal environment (e.g., Besponsa and Mylotarg), releasing cytotoxic small molecules to exert cell-killing effects. Disulfide bonds can be reduced by intracellular glutathione (GSH) to release cytotoxic payloads, with steric hindrance preventing premature cleavage before cellular uptake. If the released cytotoxic payload can penetrate tumor cell membranes, it can kill neighboring cancer cells through a "bystander effect." However, the bystander effect is not guaranteed and depends on the membrane permeability and charge properties of the released payload. It is important to note that the bystander effect can also kill normal or immune cells near the target tumor cells. Another drawback of cleavable linkers is potential metabolic degradation in circulation, leading to off-target toxicity.
Non-cleavable linkers consist of structures stable in circulation, and ADCs with these linkers release the payload only after proteolytic degradation in lysosomes. Non-cleavable linkers significantly reduce off-target toxicity caused by extracellular release but have lower release efficiency and require efficient internalization. After internalization into lysosomes, non-cleavable linkers remain intact while the antibody is degraded into amino acids, forming amino acid-linker-cytotoxic complexes. The charged "linker-amino acid residues" limit their membrane permeability and diffusion, preventing the bystander effect.
4. Cytotoxic Payloads in ADCs
The cytotoxic payload is the most critical effector component of ADCs. Common cytotoxic molecules include microtubule inhibitors, DNA-damaging agents, and DNA transcription inhibitors. Microtubule inhibitors bind to tubulin to prevent polymerization, thereby arresting the cell cycle and exerting cytotoxic effects, such as Monomethyl auristatin E and Maytansine and their analogs. DNA-damaging agents bind to the minor groove of DNA and promote alkylation, strand breaks, or cross-linking, such as Calicheamicin. DNA transcription inhibitors include Amatoxin and Quinoline Alkaloid (SN-38), such as CL2A-SN-38, a drug-linker conjugate formed by linking the linker CL2A to the cytotoxic molecule SN-38 for ADC preparation.
5. Conjugation Methods of ADCs
Conjugation methods can be divided into non-site-specific and site-specific conjugation. Early methods primarily used lysine or cysteine conjugation. Site-specific conjugation involves genetic engineering to achieve specific conjugation sites, enabling homogeneous ADCs with cytotoxic molecules linked at defined positions.
Absin ADC Toxin-Antibody Site-Directed Conjugation Kit (abs580253) offers a convenient operation without the need for complex antibody engineering, enabling rapid site-specific conjugation of monoclonal antibodies. The conjugated products are uniform and stable, suitable for site-specific conjugation during the ADC development phase and early ADC research.
Conjugation Principle:
1) Antibody Azidation

First, EndoS glycosidase is used to expose the conserved N-glycan on the Fc region of the antibody, revealing N-acetylglucosamine (blue square). Subsequently, a mutant β-galactosyltransferase (b.GalTY298L) is used to attach an azide-functionalized galactosamine (yellow square-N3) to the N-acetylglucosamine.
2) Payload Conjugation
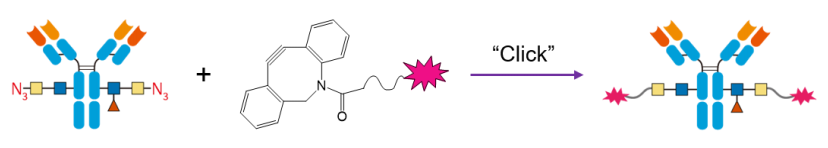
Next, a copper-free click chemistry reaction, such as SPAAC (Strain-Promoted Alkyne-Azide Cycloaddition), is used to attach biotin, fluorophores, or cytotoxic molecules to the antibody.
Absin (Shanghai) Biotechnology Co., Ltd. offers ADC cytotoxins, ADC linkers, ADC linkers with payloads, ADC site-directed conjugation kits, ADC target proteins, ADC reference antibodies, ADC overexpressing drug target cell lines, and other reagents, as well as antibody customization and mIHC services. These products and services enable researchers to accelerate ADC drug development from antibody preparation, screening, conjugation to mIHC pathological detection.
References
[1] Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct Target Ther[J]. 2022 Mar 22;7(1):93.
[2] Wu G, Fu Z, Xu G, et al. Advances in the development of antibody-drug conjugates[J]. Biomedical Transformation, 2021, 2(4):11.
Recommended ADC-Related Products:
| Catalog No. | Product Name | Specification | |
| abs580253 | ADC Toxin-Antibody Site-Directed Conjugation Kit | 500ug | |
| abs580253 | ADC Toxin-Antibody Site-Directed Conjugation Kit | 1mg | |
Hot Targets for ADC Proteins
| Catalog No. | Product Name | Specification | 规格 |
| abs04770 | Recombinant Biotinylated Human Siglec-2 Protein(C-His-Avi) | Siglec-2 | 100ug |
| abs05079 | Recombinant Human Nectin-4 Protein(C-8His) | Nectin-4 | 100ug |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
