- Cart 0
- English
IF 29.4│Tumor-Targeting Bacterial Flagella Reprogram TAMs via Local Hemorrhage
Review
Tumor-associated macrophages (TAMs) are a heterogeneous population of plastic cells in the tumor microenvironment, accounting for up to 50% of some solid tumors. TAMs typically exhibit an immunosuppressive M2 phenotype, leading to poor prognosis. TAMs promote tumor progression by stimulating angiogenesis, increasing tumor cell migration and invasion, and suppressing anti-tumor immunity. Due to the high plasticity of TAMs, polarizing anti-inflammatory macrophages to pro-inflammatory macrophages is a promising anti-tumor strategy. Current strategies commonly involve local or systemic administration of exogenous substances, such as immune factors, monoclonal antibodies, and immune agonists. However, the development of new materials that can precisely induce tumor hemorrhage without affecting normal coagulation remains challenging.
Recently, a research team led by Professor Jinhui Wu from Nanjing University published a research paper titled “Flagella of Tumor-targeting Bacteria Trigger Local Hemorrhage to Reprogram Tumor-associated Macrophages for Improved Antitumor Therapy” in ADVANCED MATERIALS (IF=29.4). Theyconstructed a tumor-targeting multi-flagellated bacterium (flhDC VNP) via genetic engineeringto achieve precise tumor hemorrhage. flhDC VNP colonizes tumors and overexpresses flagella during proliferation. The flagella promote the expression of tumor necrosis factor TNFα, leading to localized tumor hemorrhage. Erythrocytes infiltrating during hemorrhage temporarily polarize macrophages to the M1 subtype. In the presence of artesunate (Arts), a complex is formed with heme, continuously generating reactive oxygen species (ROS) to transform transient polarization into sustained polarization.Thus, the flagella of tumor-targeting bacteria may open new strategies for reprogramming TAMs and improving anti-tumor therapy.

Figure 1 Schematic diagram of tumor-targeting bacterial flagella triggering localized hemorrhage and TAM reprogramming
Construction and Identification of FlhDC VNP andΔflhD VNP
The research team first introduced a plasmid overexpressing the flagellar master regulator flhDC into the attenuated Salmonella VNP20009 (Vnp), constructing the flhDC VNP bacteria. Induced by L-arabinose, flhDC VNP successfully expressed the fluorescent protein tdTOMATO encoded by the overexpression plasmid,flagella, and flhDC protein. To investigate whether the overexpression of flagella was stable, they passaged flhDC VNP continuously, demonstrating that the overexpression of flagella in flhDC VNP was relatively stable. Scanning electron microscopy revealed that the number of flagella in flhDC VNP was significantly higher than in unedited VNP. A flagella-deficient Salmonella ΔflhD VNP was constructed by knocking out the flhD gene in the bacterial genome. Scanning electron microscopy showed no flagella on the surface of ΔflhD VNP. To study whether the number of flagella is necessary for bacterial motility, researchers conducted an agar-sealed Transwell assay. flhDC VNP could penetrate the agar within 2 hours, while VNP and ΔflhD VNP hardly penetrated. Further experiments in 3D cell tissues showed that flhDC VNP penetrated deeper. Therefore, these results indicate that they successfully constructed stable flhDC VNP and ΔflhD VNP, and bacterial motility increased with the number of flagella.
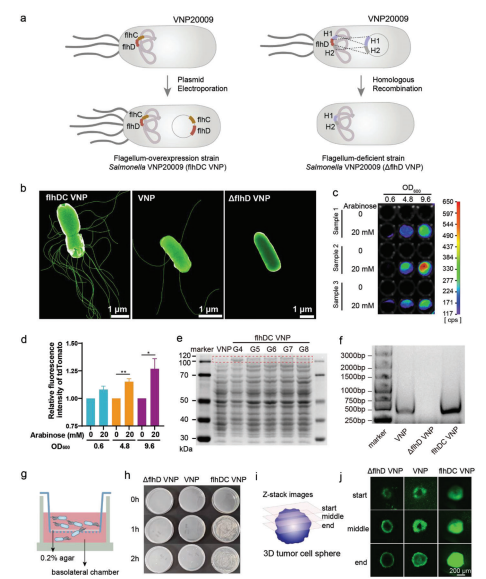
Figure 2 Construction and characterization of multi-flagellated bacteria
FlhDC VNP-Induced Tumor Hemorrhage
To investigate whether an increased number of flagella enhances bacterial accumulation in tumors, researchers intravenously injected equal amounts of VNP, flhDC VNP, and ΔflhD VNP into tumor-bearing mice. Major organs (heart, liver, spleen, lung, kidney) were harvested at different time points for bacterial counting. Results showed that bacterial accumulation in tumors increased over time for all strains. Moreover, 72 hours post-injection, flhDC VNP accumulated 10-fold more in tumors than VNP and ΔflhD VNP. These data indicate that an increased number of flagella facilitates bacterial proliferation within tumors. In other organs, bacterial distribution declined gradually due to immune cell clearance.
To explore whether an increased number of flagella enhances the secretion of inflammatory cytokines in the blood, they compared the concentration of tumor necrosis factor TNFα over time after intravenous injection of flhDC VNP, ΔflhD VNP, and VNP. Results showed that TNFα concentration peaked 1 hour post-injection, with flhDC VNP stimulating significantly higher TNFα secretion than ΔflhD VNP and VNP. Due to the low flow rate and high permeability of tumor vasculature, excessive inflammatory cytokines may disrupt tumor vessels. Over time, tumor hemorrhage worsened, with maximal erythrocyte infiltration at 12 hours post-injection. Tumors were harvested 12 hours post-injection and stained with hematoxylin and eosin (H&E). Tumors injected with flhDC VNP contained the most infiltrating erythrocytes. These data demonstrate that flhDC VNP accumulates in tumors, inducing hemorrhage through increased inflammatory cytokine levels.
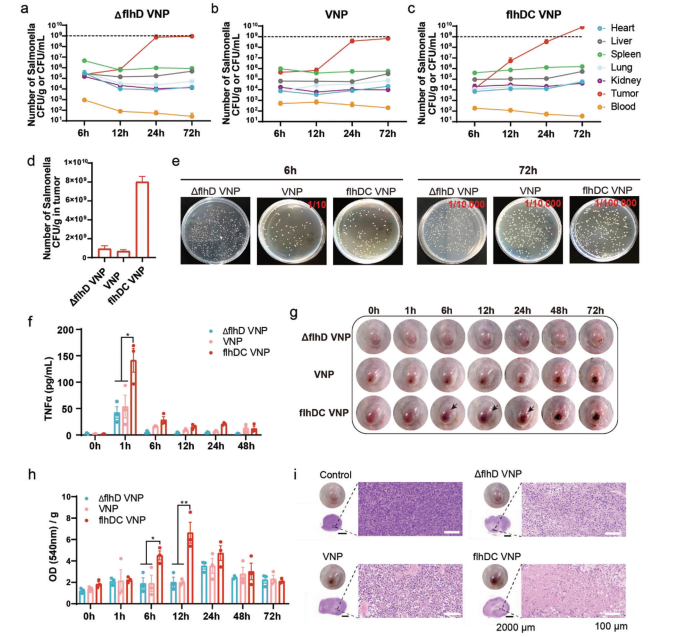
Figure 3 Bacteria-induced TNFα secretion and tumor hemorrhage
Heme and Arts Reaction Form a Complex Capable of Polarizing Macrophages
Under normal conditions, infiltrating erythrocytes are phagocytosed by macrophages. Within macrophages, erythrocytes are degraded into hemoglobin, which is further digested into heme. Heme is then transported from endosomes to the cytoplasm of macrophages via HRG1. Heme can react specifically with antimalarial drugs containing peroxide bonds, such as Arts. When Arts is activated by a low-valent metal complex (heme), the weak peroxide bond undergoes homolytic cleavage, generating reactive oxygen species (ROS). Researchers found that heme and Arts form a peroxidase-active complex, which can catalyze the production of hydroxyl radicals from hydrogen peroxide and similarly increase intracellular ROS levels.
Increased ROS levels affect macrophage phenotype. ROS promote pro-inflammatory macrophage polarization through the MAPK signaling pathway, NF-kB signaling pathway, NOD-like receptors, and changes in mitochondrial membrane potential. Research results indicate that the Arts-heme complex significantly enhances the pro-inflammatory phenotype of macrophages. Pro-inflammatory regulation is mediated by changes in mitochondrial membrane potential. Therefore, the complex formed by erythrocytes and Arts increases intracellular ROS levels in macrophages, polarizing TAMs through changes in mitochondrial membrane potential and activation of the NLRP3 inflammasome.
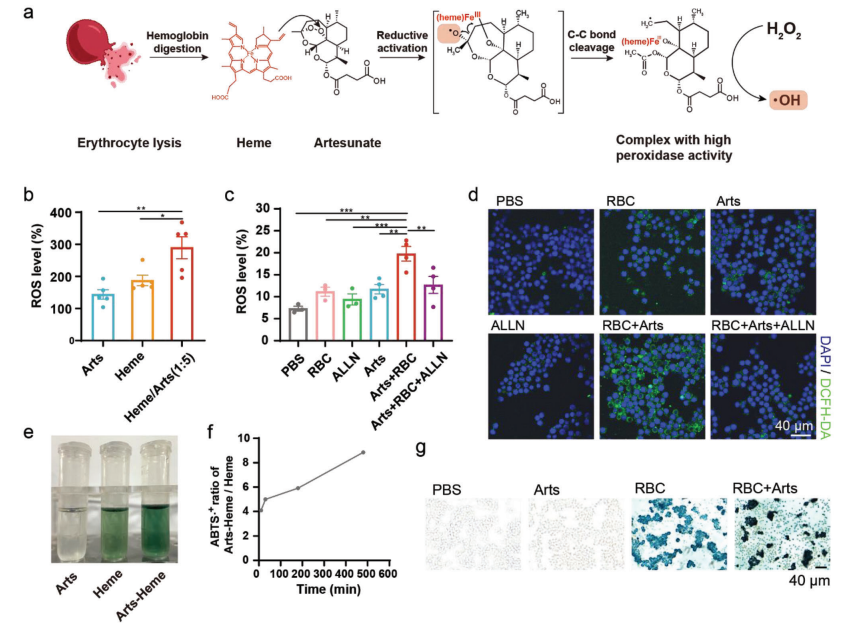
Figure 4 Heme and Arts reaction generates ROS to sustain macrophage polarization
FlhDC VNP Combined with Arts Polarizes Macrophages and Promotes Tumor Inflammatory Microenvironment
Given that macrophages can account for up to 50% of the tumor and exhibit high plasticity, the research team further investigated the phenotypic changes of TAMs following combined treatment with flhDC VNP and Arts. Results showed that the combination of flhDC VNP and Arts significantly increased the proportion of pro-inflammatory TAMs and decreased anti-inflammatory TAMs. Pro-inflammatory macrophage polarization may alter the tumor immune microenvironment. Transcriptomic analysis of tumors revealed that flhDC VNP and Arts upregulated multiple inflammation-related genes and various inflammatory signaling pathways, chemokine pathways, and immune system process pathways, such as TNF signaling, NF-κB signaling, and chemokine signaling pathways. Therefore, the combination of flhDC VNP and Arts polarizes TAMs to a pro-inflammatory phenotype, increases immune cell infiltration in tumors, and transforms the tumor immune microenvironment from “cold” to “hot”.
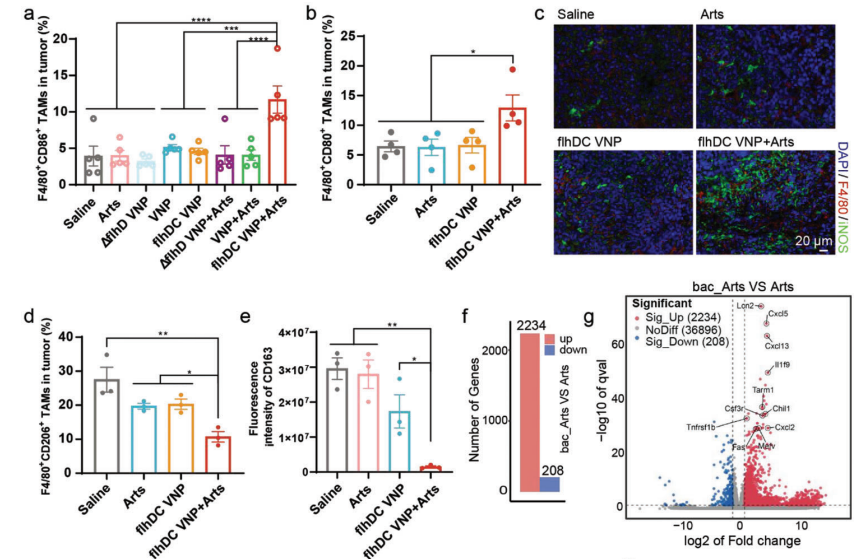
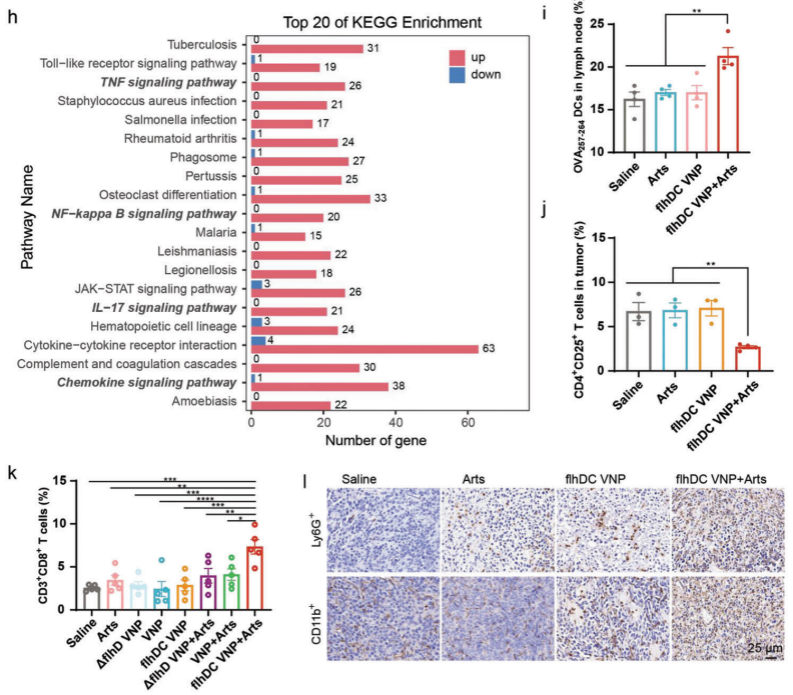
Figure 5 Characterization of tumor immune microenvironment following combined treatment with FlhDC VNP and Arts
Anti-tumor Effects of FlhDC VNP Combined with Arts in Different Tumor Models
Finally, the research team evaluated the anti-tumor effects of flhDC VNP in a CT26 xenograft tumor model via intravenous injection of bacteria combined with intraperitoneal injection of Arts. Results showed that the anti-tumor effect of Arts was enhanced when combined with flhDC VNP. Additionally, to better simulate the tumor growth environment, an orthotopic colon tumor model was used to assess anti-tumor effects. Results confirmed that flhDC VNP enhanced the anti-tumor effect of Arts. Further research demonstrated that the combination of flhDC VNP and Arts exhibited anti-tumor effects in CT26 xenografts, orthotopic CT26 colon cancer, and chemotherapy-resistant melanoma, and enhanced the anti-tumor effect of TCR-T cell therapy.
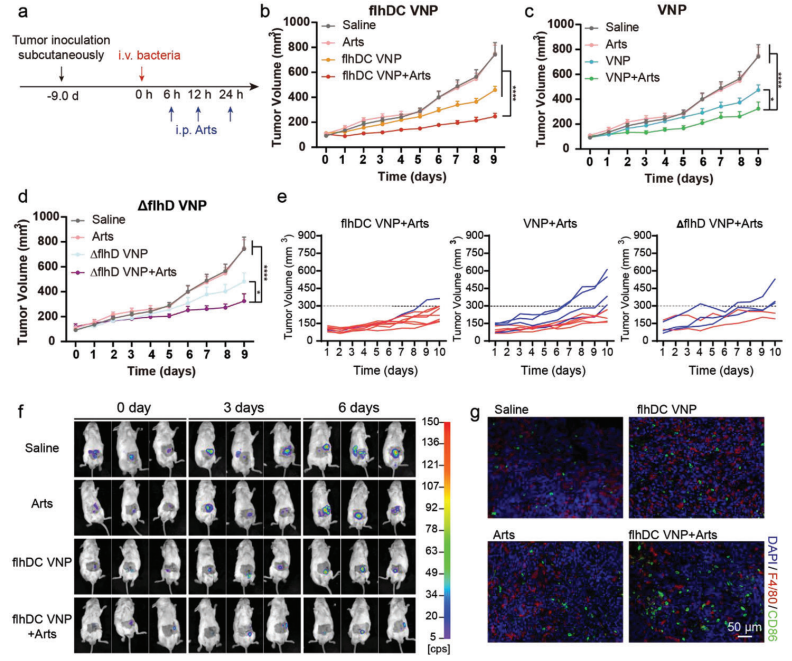
Figure 6 Anti-tumor effects of FlhDC VNP combined with Arts
In summary, tumor-targeting bacterial flagella may open new strategies for reprogramming TAMs and remodeling the tumor immune microenvironment to improve anti-tumor therapy. This research has broad application prospects and is supported by the National Key Research and Development Program and the National Natural Science Foundation of China.
Did You Know? Tumors are always at the top of the research hotlist. Besides being classified as “benign” or “malignant”, tumors also have “cold” and “hot” distinctions. Based on the spatial distribution of immune cells in the tumor microenvironment, tumors are categorized into three basic immune phenotypes: immune-inflammatory, immune-excluded, and immune-desert. Immune-inflammatory tumors are referred to as “hot tumors”, while immune-excluded and immune-desert tumors are collectively known as “cold tumors”. The aforementioned study utilized L-arabinose to promote the expression of exogenous genes in attenuated Salmonella. In addition to this, Absin offers a wide range of high-quality small-molecule agonists/inhibitors and biochemical reagents related to tumor research, assisting researchers in continuously innovating and breaking through on their scientific journey!
References
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
February 19, 2025
Clicks:261
