- Cart 0
- English
Organoid HE Staining, IHC, and IF Identification
How can we identify the organoids we have painstakingly cultivated? Typically, this involves HE staining, immunohistochemistry, or immunofluorescence. Today, let Xiao Ai guide you through the specific staining procedures.
I. HE Staining Experimental Steps
1. Collection of Organoids

(1) Organoid Collection (e.g., collect organoids from 6-8 wells of a 24-well plate, approximately 1000-2000 organoids)
(2) Organoid Washing
Re-suspend in 1ml PBS and transfer to a 1.5ml centrifuge tube, centrifuge at 300g at 4°C for 5 minutes and discard the liquid.
2. Organoid Fixation
(1) PFA Fixation
Add 1ml of 4% paraformaldehyde (abs9179) to an EP tube, gently resuspend to mix, and fix at 4°C for 1 hour (not exceeding 10 hours). After fixation, centrifuge at 300g at 4°C for 5 minutes and discard the supernatant.
(2) Organoid WashingRe-suspend in 1ml PBS and transfer to a 1.5ml centrifuge tube, centrifuge at 300g at 4°C for 5 minutes and discard the liquid.
3. Organoid Agarose Embedding
(1) Weigh 0.2 to 0.4g of agarose and place it in a beaker or small reagent bottle, add about 10ml of PBS. Melt in a microwave at high power, pausing every 5 seconds to check, repeat several times until the agarose is completely melted.
(2) Once the agarose solution cools to 50-60°C, take 20-50ul of the melted agarose solution and add it to a 1.5ml EP tube to re-suspend the organoid pellet, place on ice for 30 minutes until it solidifies.
4. Organoid Paraffin Embedding
(1) Dehydration: Remove the solidified agarose block containing the organoid pellet and dehydrate in a graded ethanol series, 70%, 80%, 90%, and 95% ethanol for 1 hour each, and 100% ethanol for 30 minutes until it solidifies;
(2) Wax Melting: Preheat the oven to 65°C about 4 hours in advance to melt the paraffin;
(3) Clarification: Transfer the dehydrated agarose block to the tissue embedding box (keep it immersed in absolute ethanol until treated with xylene), transfer the box to xylene for 5 minutes for two treatments; Note: Xylene is toxic, so perform the operation in a well-ventilated area and try to drain any residual xylene before removing it.
(4) Infiltration: Immediately after clarification, transfer to the melted wax container, infuse with wax at 60°C for 2 hours, then change to a new wax container and infuse again for 2 hours;
(5) Embedding: Perform embedding on an ice box, pour the melted pure wax into the wax box to one-third full, place the agarose block in the middle, and then drip wax for embedding until full. Carefully remove the mold after the wax block is completely solidified.
(6) Sectioning: Secure the embedded paraffin block in the microtome and cut thin sections, typically 5-8um thick, and use small forceps to place the sections containing intact tissue into warm water at 40°C;
(7) Collecting Tissue: The tissue expands upon heating, ideally without wrinkles. When collecting tissue with a glass slide, generally take the lower 1/3 or 1/2 of the slide. Typically, collect 5-6 slides per tissue type, with 2-3 as backups. Each slide usually carries two tissue samples for comparison, which reduces the error. It's best to maintain consistent orientation when collecting the slides for easier observation. Then place the collected slides on a rack and put them into a 37°C incubator to dry.
5. Organoid HE Staining
(1) Deparaffinization to Water: Sequentially place the sections in Xylene I for 10min; Xylene II for 10min; Anhydrous Ethanol I for 5min; Anhydrous Ethanol II for 5min; 95% Alcohol for 5min; 90% Alcohol for 5min; 80% Alcohol for 5min; 70% Alcohol for 5min; Rinse with distilled water;
(2) Hematoxylin Staining of Cell Nuclei: Immerse the sections in Harris Hematoxylin for 3-8 minutes, and rinse with tap water;
(3) Eosin Staining of Cytoplasm: Immerse the sections in Eosin staining solution for 1-3 minutes, and rinse with tap water;
(4) Dehydration and Mounting: Sequentially place the sections in 95% Alcohol I for 5min; 95% Alcohol II for 5min; Anhydrous Ethanol I for 5min; Anhydrous Ethanol II for 5min; Xylene I for 5min; Xylene II for 5min to dehydrate and clarify. Remove the sections from Xylene and let them air dry slightly, then mount with neutral balsam;
(5) Microscopic Examination, Image Capture, and Analysis.
6. Organoid Authentication - HE Staining Results (Lung Cancer as an Example)
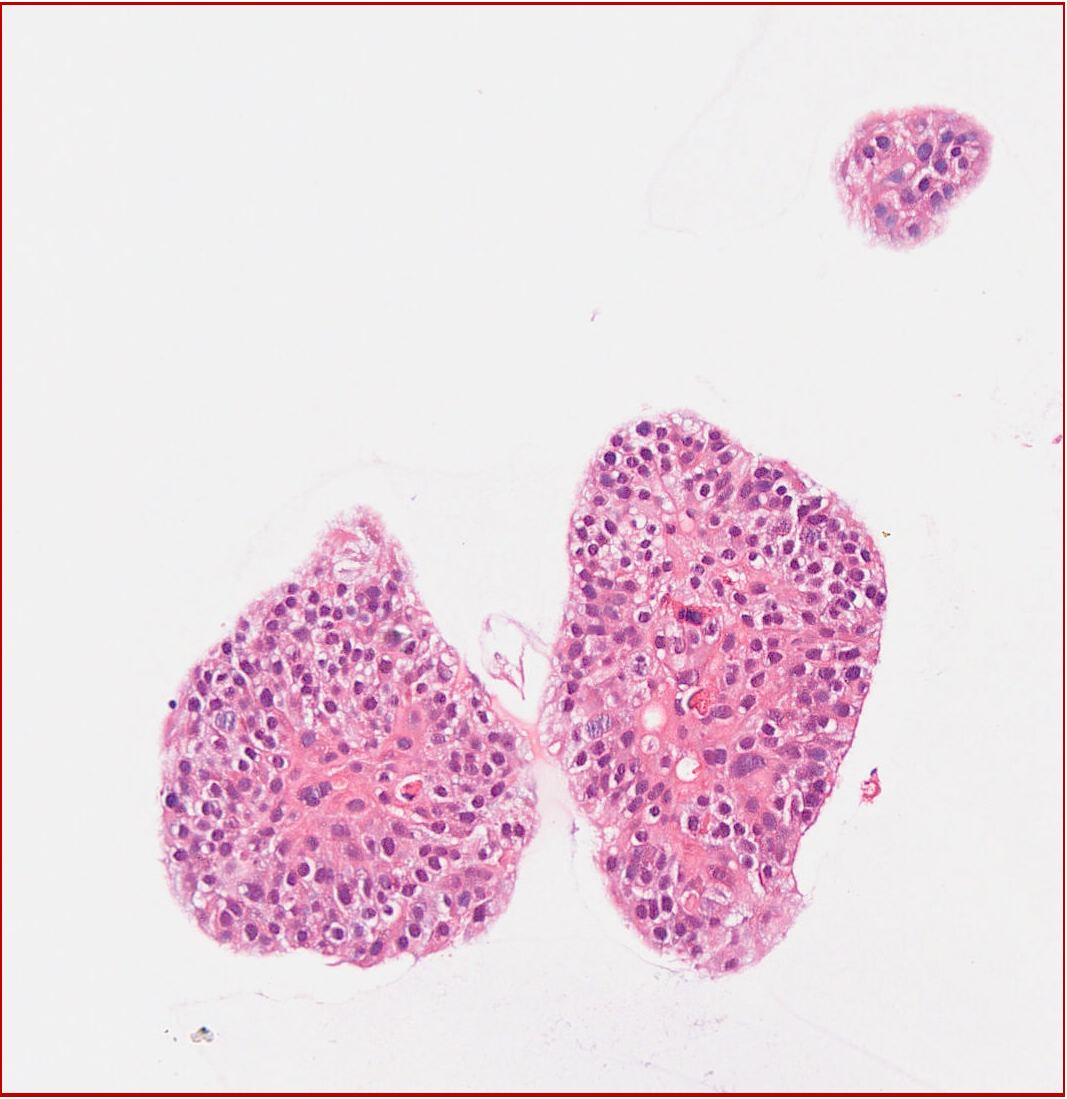
Human Lung Cancer Organoid HE Staining
II. Immunohistochemistry Experimental Procedures
Process the sections that have been deparaffinized in the HE staining steps as follows:
1. Antigen Retrieval: Soak the sections in 3% H2O2 for 10 minutes to remove endogenous peroxidases, then discard the H2O2 and wash twice in water. Add citrate buffer and place in a microwave for 3 minutes at medium heat until it begins to boil, then allow it to cool to room temperature. Repeat the microwaving process, allowing it to cool to room temperature again. The purpose of microwaving is to expose the antigen sites.
2. Serum Blocking: After cooling to room temperature, discard the citrate buffer, wash twice, and place the slides in PBS for 5 minutes, then wash twice. Wipe away the PBS around the tissue, immediately add serum to block non-specific sites, and then place in a 37°C incubator for half an hour. Serum is diluted 10-fold (900µl PBS: 100µl serum blocking solution).
3. Add Primary Antibody: Remove the slides from the incubator, wipe the back and front of the slides with absorbent paper to remove any serum, and add the primary antibody. If a control experiment is being done, add PBS to the control tissue. After adding the primary antibody, store the slides overnight in a 4°C refrigerator.
4. Add Secondary Antibody: Remove the slides from the refrigerator, wash in PBS three times for 5 minutes each, wipe away the PBS around the tissue, add the secondary antibody, and then place in a 37°C incubator for half an hour.
5. Add SABC: Remove the slides from the incubator, wash in PBS three times for 5 minutes each, wipe away the PBS around the tissue, add SABC, and then place in a 37°C incubator for half an hour. SABC is diluted 100-fold (990µl PBS: 10µl SABC).
6. Add Chromogen: Remove the slides from the incubator, wash in PBS three times for 5 minutes each, wipe away the PBS around the tissue, and add the chromogen. (Chromogen preparation: Add 1 drop of chromogen A to 1ml of water, mix well, then add 1 drop of chromogen B, mix well, and then add 1 drop of chromogen C, mix well, A: DAB, B: H2O2, C: Phosphate-buffered saline).
7. Counterstaining: After the development, rinse the slides in water for a while, then immerse in hematoxylin for staining for half a minute.
8. Dehydration: After counterstaining, rinse the slides in water, and then place the slides sequentially in 70% alcohol - 80% alcohol - 90% alcohol - 95% alcohol - 100% alcohol - 100% alcohol - xylene - xylene. Place for 2 minutes in each reagent, and finally soak in xylene, then move to a fume hood.
9. Mounting: Apply neutral balsam around the tissue, then cover with a cover slip, first lay flat one side, and then gently place the other side to avoid air bubbles. After the slides are mounted, place them in a fume hood to dry.


III. Immunofluorescence Experimental Procedures
Process the sections after the primary antibody incubation in the immunohistochemistry steps as follows:
1. Add Fluorescent Secondary Antibody: Wash the sections with PBS three times for 3 minutes each. After removing excess liquid, add the diluted fluorescent secondary antibody and incubate in a moist chamber at 37°C for 1 hour, then wash with PBS three times. Note: From the addition of the fluorescent secondary antibody onwards, all subsequent steps should be performed in minimal light conditions to avoid bleaching.
2. Nuclear Counterstaining: Add DAPI and incubate in the dark for 5 minutes to stain the nuclei. Wash with PBS four times to remove excess DAPI.
3. Mounting: Mount the sections with an anti-fade mounting medium and observe under a fluorescence microscope.
|
Item NO. |
Product Name |
Size |
|
1kit |
||
|
1kit |
||
|
Mouse Lung Organoid Culture Medium Kit |
1kit |
|
|
Human Breast Cancer Organoid Culture Medium Kit |
1kit |
|
|
Human Endometrial carcinoma Organoid Culture Medium Kit |
1kit |
|
|
Human Colorectal cancer Organoid Culture Medium Kit |
1kit |
|
|
Human Lung Cancer Organoid Culture Medium Kit |
1kit |
|
|
1kit |
||
|
Human Pancreatic Cancer Organoid Culture Medium Kit |
1kit |
|
|
Human Ovarian Cancer Organoid Culture Medium Kit |
1kit |
|
|
Human Liver Organoid Culture Medium Kit |
1kit |
|
|
1kit |
||
|
Mouse Gastric Cancer Organoid Culture Medium Kit |
1kit |
|
|
1kit |
||
|
Mouse Breast Cancer Organoid Culture Medium Kit |
1kit |
|
|
Mouse Hepatocarcinoma Organoid Culture Medium Kit |
1kit |
|
|
1kit |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
October 24, 2024
Clicks:472
