- Cart 0
- English
A Guide to Cultivating Organoids of Colorectal Cancer
October 17, 2024
Clicks:480

I. Preparation Work
1. Equipment
CO2 Incubator, Dual-sided Laminar Flow Cabinet, Inverted Microscope, Benchtop Refrigerated Centrifuge, Water Bath (abs72023) or Water Bath Shaker, Medical Refrigerator, -80°C Freezer, Pipettes (a set), Ophthalmic Scissors, Ophthalmic Forceps.
2. Reagents and Consumables
Human Colorectal Cancer Organoid Culture Medium Kit (abs9445), Matrix Gel (Low Factor, Phenol Red-Free) (abs9495), 60mm Cell Culture Dish (abs7005), 100μm Filter Screen (abs7009), 15ml Centrifuge Tube (abs7102), 1.5ml EP Tubes (several) (abs7119), 24-Well Cell Culture Plate (abs7058), Metal Ice Box, Ophthalmic Scissors, Ophthalmic Forceps.
II. Operational Procedure
1. Organoid Operation Procedure - Sample Preparation
(1) Place the tissue into a sampling vial containing pre-cooled (2-8°C) tissue preservation solution E (submerge the entire tissue), and retrieve it from the hospital/laboratory at 4°C.
(2) Disinfect the sampling vial, remove the tissue, place it into a culture dish, take a photo of the sample, and record information such as size, color, texture, hardness, and type of tissue.

2. Organoid Operation Procedure - Washing and Mincing
Soak in a 60mm cell culture dish (abs7005) with 2-3ml of primary culture buffer B. After washing three times with primary culture buffer B (changing the culture dish each time), mince the tissue into pieces approximately 1-3mm^3 in size, and let it stand for 2 rounds.

3. Organoid Operation Procedure - Digestion and Filtration
(1) Add an appropriate amount of human colorectal cancer primary tissue digestion solution C (the digestion solution should be 3-5 times the volume of the tissue) to an EP tube and digest at 37°C for 10-15 minutes (observe the digestion process at any time).
(2) Take a small amount of the liquid and observe it under a microscope. Once a majority of single cells or cell clusters smaller than 70μm are observed, add 3 times the volume of Primary Culture Buffer B to terminate the digestion. Gently pipette the solution to see it become turbid.
(3) Filter the digested tissue using a 100μm filter screen (abs7009), and observe a small amount of the filtrate under a microscope. Collect the filtrate into a 15ml centrifuge tube and perform a centrifugation enrichment at 300g at 4°C for 5 minutes to remove the supernatant (washing once). Add approximately 1ml of Primary Culture Buffer B and transfer to a 1.5ml EP tube, then resuspend and centrifuge again (washing twice).


4. Organoid Operation Procedure - Centrifugation and Matrix Gel Addition
Matrix Gel Calculation: After step 3, observe and measure the volume of the collected tissue, then add 25 times the volume of Matrix Gel (abs9495) to resuspend and plate the tissue (perform operations on a metal ice box or on ice).
Using a 24-well cell culture plate as an example, spot 25μl of the tissue-matrix gel mixture per well to plate (perform operations on a metal ice box or on ice). Place the plated culture plate into a 37°C incubator for 10-15 minutes to allow the gel to set, then add 500-750μl of Mouse Normal Liver Organoid Culture Medium A (which has been returned to room temperature) for cultivation.

6. Organoid Operation Procedure - Observation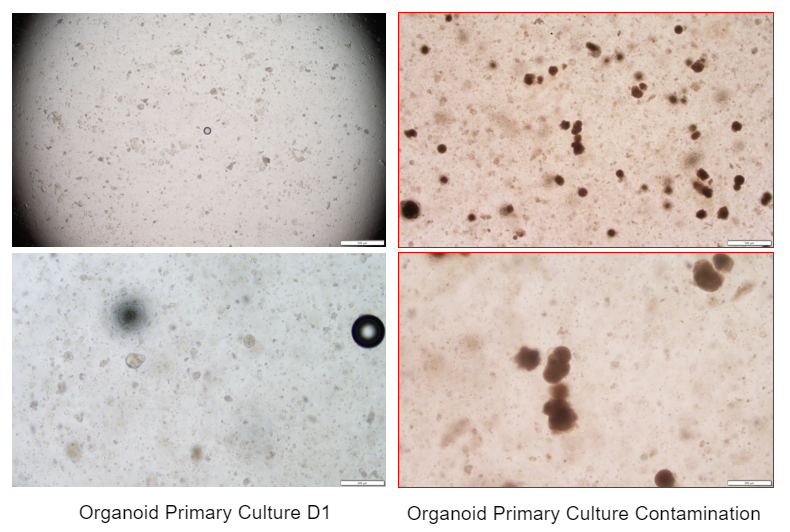

I. Organoid Passaging Culture - Sample Collection
1. Use a pipette to remove the culture medium, and add approximately 1-2ml of 4°C Organoid Passaging Buffer G to each well, letting it sit for 2 minutes.
2. Gently pipette the matrix gel and collect it in a 15ml centrifuge tube, allowing it to stand at 4°C for 10 minutes (grouping every 6-8 wells).
II. Organoid Passaging Culture - Sample Digestion
1. When the number of organoids is low or their volume is small: Centrifuge at 300g for 5 minutes at 4°C, discard the supernatant, add 1ml of Organoid Passaging Buffer G to resuspend, and transfer into a 1.5ml centrifuge tube. Centrifuge at 300g for 5 minutes at 4°C (as shown in Figure 5), discard the liquid, and proceed to step 3.
2. When the number of organoids is high or their volume is large: Centrifuge at 300g for 5 minutes at 4°C, discard the supernatant, add an appropriate amount of Organoid Digestion Liquid D (1-2 times the volume of the organoid suspension) and digest on a super clean bench for 2-3 minutes (you can pipette gently 1-2 times during the process) (as shown in Figure 6). Add an appropriate amount of Organoid Passaging Buffer G to terminate the digestion (the ratio of Buffer G to Digestion Liquid D should be 5:1). Centrifuge at 300g for 5 minutes at 4°C, discard the supernatant, add 1ml of Organoid Passaging Buffer G to resuspend and transfer into a 1.5ml centrifuge tube (as shown in Figure 7). Centrifuge at 300g for 5 minutes at 4°C, discard the liquid, and proceed to step 3 (as shown in Figure 4).
III. Organoid Passaging Culture - Plating
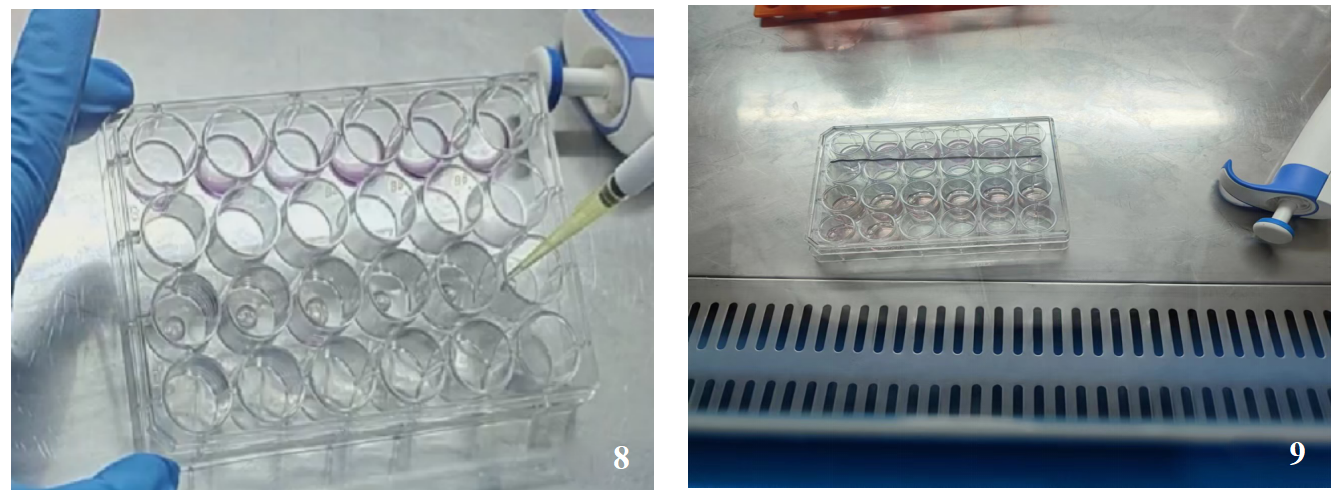
After collecting the organoids, add 25 times the volume of Matrigel to resuspend (the ratio of Matrigel volume to cell pellet volume is 25:1), with 25 μl per well (as shown in Figure 8). Spread the Matrigel in a 24-well cell culture plate and let it set in the incubator for 10-15 minutes. Then, add 500 μl to 750 μl of human colorectal cancer organoid culture medium A (as shown in Figure 9).
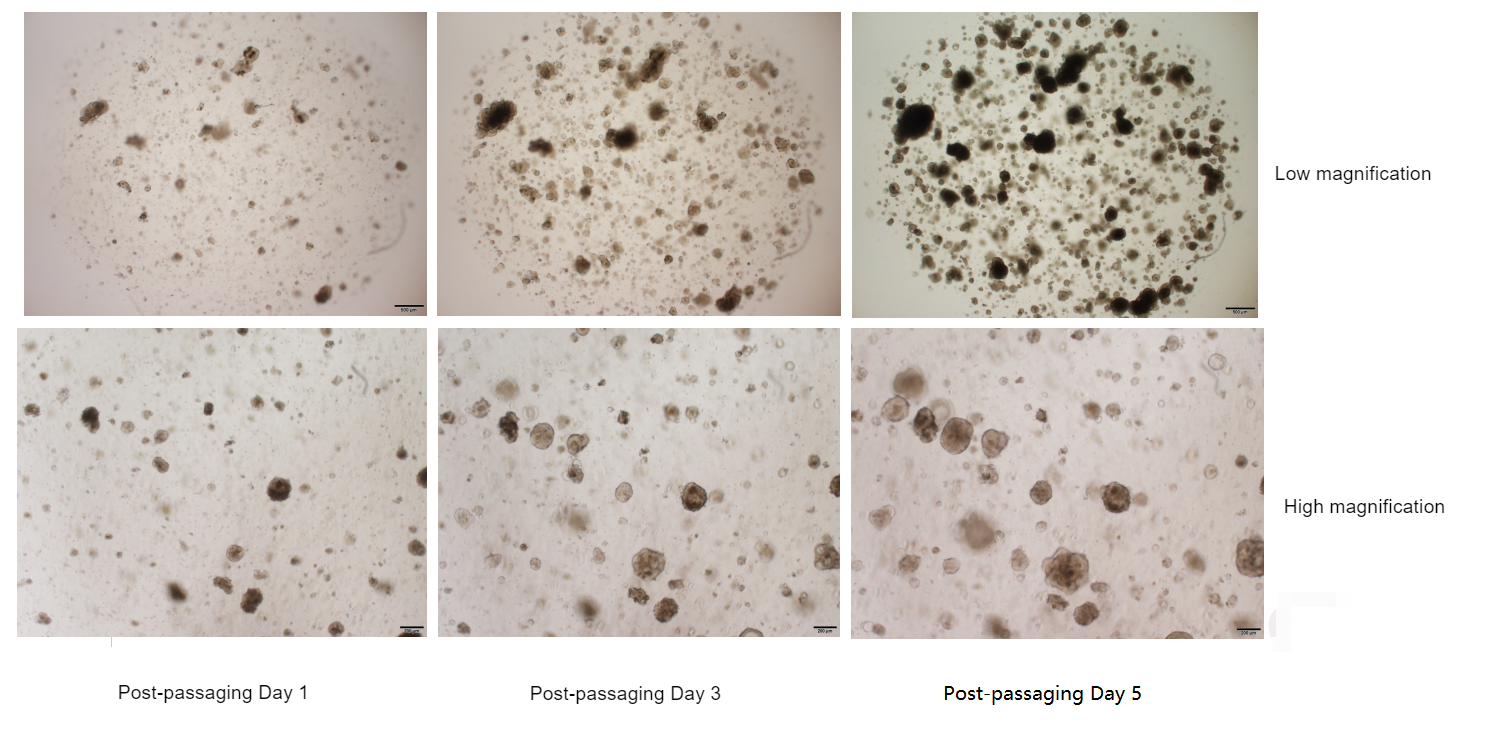
IV. Proliferation Phenomenon After Organoid Passaging


1. Use a pipette to remove the culture medium, and add approximately 1-2ml of 4°C organoid passaging culture buffer G to each well, let it sit for 2 minutes.
2. Gently pipette the matrix gel to dislodge it, collect it in a 15ml centrifuge tube, and let it stand at 4°C for 10 minutes (group every 6-8 wells as one set).
3. Centrifuge at 300g for 5 minutes at 4°C, discard the supernatant, add 1ml of organoid passaging culture buffer G to resuspend, and transfer into a 1.5ml centrifuge tube, centrifuge at 300g for 5 minutes at 4°C (as shown in Figure 5), and discard the liquid.
4. Add an appropriate amount of organoid cryopreservation solution F, gently pipette to resuspend, using a 24-well cell culture plate as an example: the density is 2 wells cryopreserved in 1 tube (cryopreservation at a density of 500 cells/ml), with a volume of 1.4ml per tube.
5. Cryopreservation methods:
(1) Place the cryopreservation tubes in a gradient freeze box and then store them at -80°C overnight, remove and place them in a liquid nitrogen tank the next day.
(2) Place in a 4°C refrigerator for 30 minutes, transfer to -20°C for 1 hour, and then move to -80°C overnight, remove and place them in a liquid nitrogen tank the next day.

1. Preparation Before the Experiment
(1) Preheat the water bath to 37°C.
(2) Perform routine disinfection in the cell laboratory by spraying with a disinfectant and exposing the surface of the laminar flow cabinet to UV light for 40 minutes.
(3) Arrange the sterilized centrifuge tubes, pipettes, culture plates, and other materials in order on the laminar flow cabinet.
2. Retrieving Cryopreserved Vials
(1) Locate the required cell vials by their labels according to the cell cryopreservation records.
(2) Remove the cryopreservation box from the liquid nitrogen tank, take out the required vials, and verify the numbers on the outside of the vials.
3. Rapid Thawing
Quickly immerse the cryopreserved vial into the preheated water bath at 37°C for thawing, and continuously agitate the vial to ensure rapid melting of the contents. After approximately 1-2 minutes, when the liquid inside the vial is completely thawed, remove the vial and wipe the outer wall with an alcohol-soaked cotton ball before taking it into the laminar flow hood.
4. Transfer the organoid suspension to a 15ml centrifuge tube, add 10ml of Organoid Passaging Culture Buffer G to resuspend, gently pipette to mix well, and centrifuge at 300g for 5 minutes at 4°C.
5. Discard the supernatant, add 1ml of Organoid Passaging Culture Buffer G to resuspend the pellet, and transfer it to a 1.5ml EP tube. Centrifuge at 300g for 5 minutes at 4°C, and discard the supernatant.
6. Add 25 times the volume of Matrigel (abs9495) to resuspend the cells (the ratio of Matrigel volume to cell pellet volume is 25:1), with 25 μl of Matrigel per well spread in a 24-well cell culture plate. Place the plate in the incubator for 10-15 minutes, then add 500 μl to 750 μl of human colorectal cancer organoid culture medium A.


I. Organoid Identification - Organoid Collection

1. Collect the organoids;
2. Wash 1-3 times to remove most of the matrix gel, centrifuge and discard the majority of the supernatant.
II. Organoid Identification - Preparation of Agarose Wells
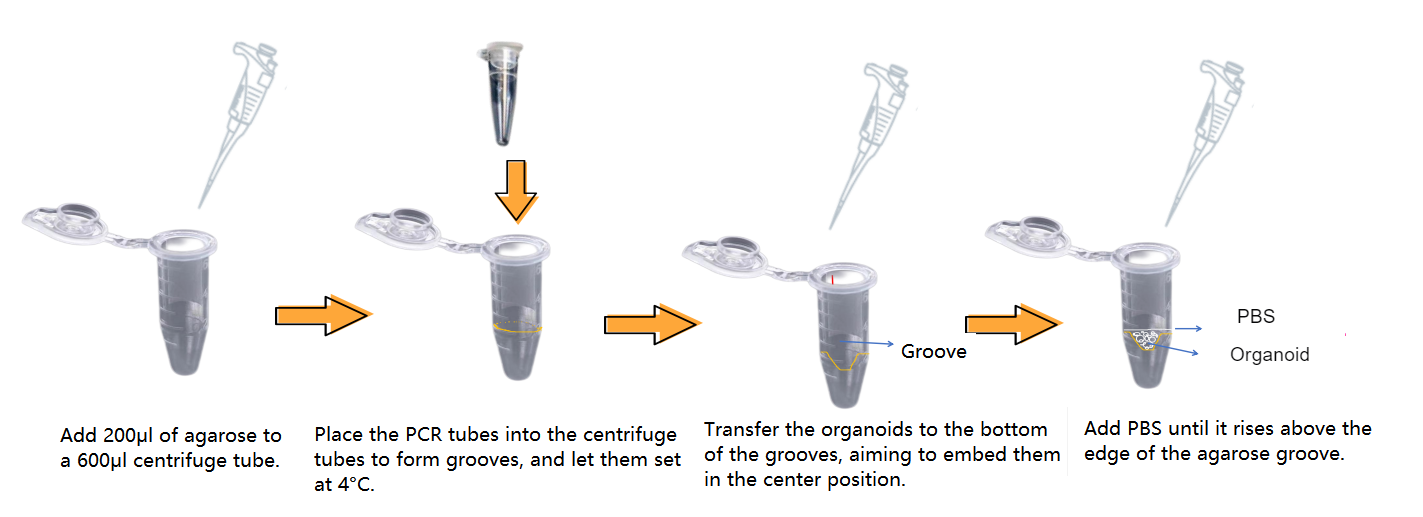
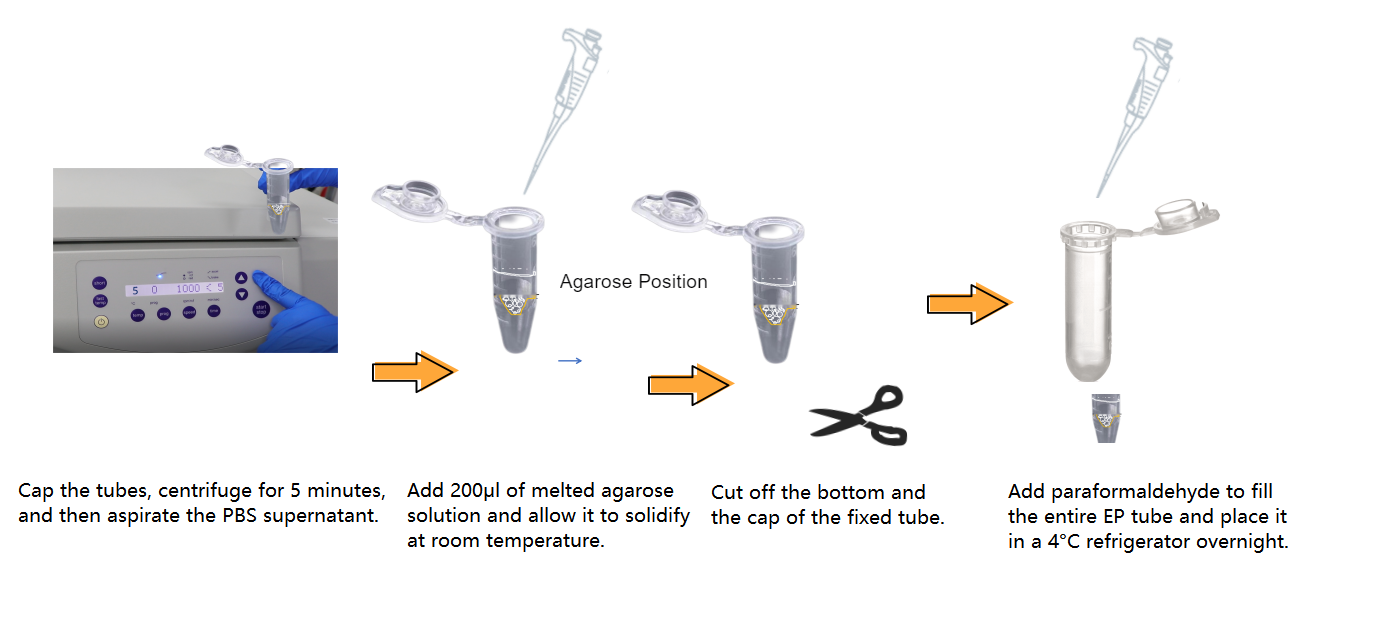
III. Organoid Identification - Fixation
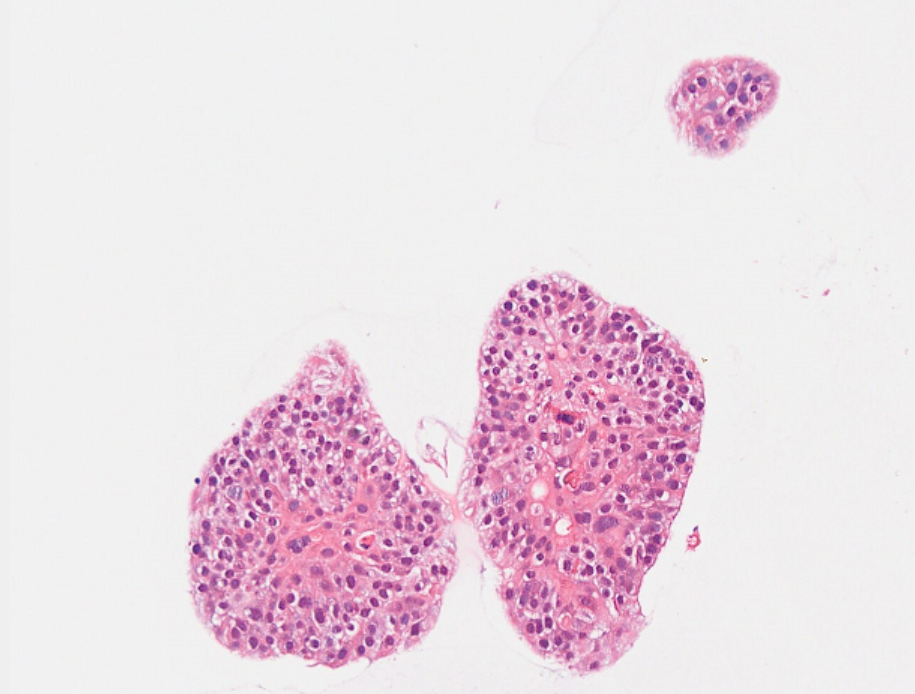
IV. Organoid Identification - HE Staining Results (with Lung Cancer as an Example)
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
