- Cart 0
- English
Organoid Culture Guide
September 13, 2024
Clicks:1016
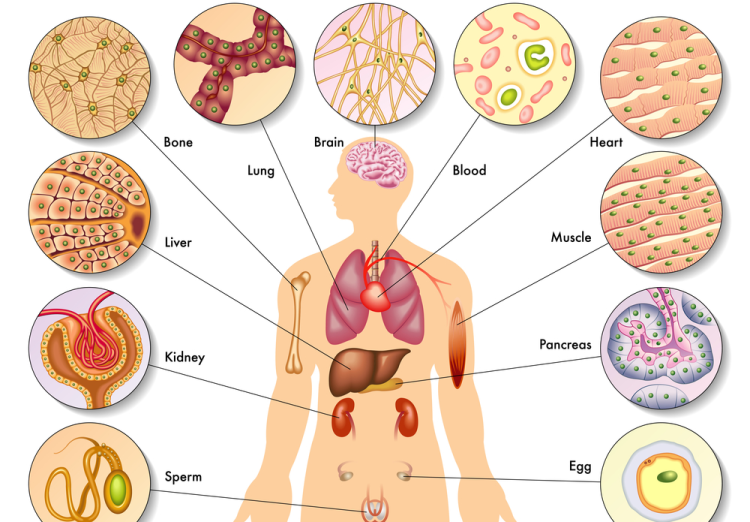
History of Organoids
As early as the 1980s, the term "organoid" had already been proposed. In 1907, Professor Wilson from the University of Bakroreina in the United States discovered that mechanically separated sponge cells could re-aggregate and self-organize into new sponge organisms with normal functions, which was the source of the future development of organoid technology. Wilson's research proved that adult organisms possess complete information and can successfully develop into new organisms without external assistance and without starting from specific anatomical stages. The contemporary development of organoids has mainly concentrated in the last decade. In 2009, Hans Clevers' laboratory used single mouse LGR5+ intestinal stem cells to self-organize into intestinal organoids with crypt-villus structures in vitro, opening a new chapter in the development of organoids.
At present, organoids of various organs have been successfully constructed, including those of the small intestine, stomach, colon, lung, bladder, brain, liver, pancreas, kidney, ovary, esophagus, heart, etc., encompassing not only normal organ tissue organoids but also corresponding tumor tissue organoids. In recent years, the number of publications involving organoid research has been rising sharply, including numerous articles in top-tier journals such as CNS. The global ranking of China's organoid publications has jumped from sixth place (2009-2019) to second place (2020), following the United States. In 2021, organoids were listed as a key special project in the "14th Five-Year Plan" for national key research and development plans. With continuous in-depth research, China's organoid technology level will be further improved, and the marketization of organoids will be increasingly enhanced under the active layout of related enterprises.

Development Process of Organoid Research
Comparison of Organoid Models with Other Models
| Feature | 2D Cell Model | Organoid | PDX |
| Physiology | Limited Model | Semi-physiological Model | Physiological Model |
| Construction Time | Short | Moderate | Long |
| Modeling Success Rate | High | High | Low |
| Drug Testing Throughput | Very High | High | Low |
| Cost | Low | Moderate | High |
| Sample Size Requirement | Small | Small Biopsy/Surgical Tissue | Large Biopsy/Surgical Tissue |
| Vascularization, Immune System | Not Available | Not Available | Available |
| Operational Difficulty | Easy | Easy | Difficult |
| Genetic Editing | Easy | Easy | Difficult |
| Tumor Heterogeneity | Difficult to Achieve | Retainable | Difficult to Achieve |
| Clinical Relevance | Low | High | High |
| Biobank Establishment | Feasible | Feasible | Difficult to Achieve |
Organoid Culture
|
Name |
Parameters |
Notes |
|
Matrix Gel |
|
Thaw in advance |
|
Basic Culture Medium |
B27 50x; N2 100x; N-Acetyl-L-cysteine 1mM; Glutamax 100x; HEPES 10mM; Primocin 500x; Advanced DMEM/F12, ADMEM; Cytokines: Mouse-EGF (Mouse epidermal growth factor) 50ng/ml; Mouse R-spondin1 500ng/ml; Mouse-noggin (BMP inhibitor) 100ng/ml |
Do not prepare too much culture medium at once, it is recommended to prepare 20ml at a time. After preparation, filter with a 0.22um filter (the purpose of filtering is sterilization). It can be stored at 4°C for 4 weeks. |
| 24-Well Cell Culture Plate |
|
|
|
PBS |
|
Sterilized, pre-cooled, and supplemented with double antibodies |
1. Dissect a 6-8 week old mouse, take approximately 10cm of the small intestine below the stomach, remove the mesentery with tweezers, and cut open the intestine along its length with scissors. Wash with pre-cooled PBS 3-5 times, carefully scrape off the villi from the surface of the small intestine using a glass slide (examine under a microscope), and place the scraped intestine into a 50ml centrifuge tube, keeping it on ice.
2. Transfer the small intestine to a biosafety cabinet and wash with PBS containing double antibiotics 3-5 times. Transfer the small intestine to a 25ml solution of 2mmol/L EDTA and incubate at 4°C for 30 minutes.
3. After digestion, transfer the small intestine to a 50ml centrifuge tube and wash twice with PBS containing double antibiotics.
4. Add 25ml of PBS and gently shake 30-50 times, take a portion of the suspension for microscopic examination. Filter the suspension through a 70um filter screen into a new 50ml centrifuge tube.
5. Repeat step 4, and centrifuge the collected filtrate at 800rpm for 5 minutes to collect the precipitate.
6. Discard the supernatant, resuspend the pellet with 2ml of ADMEM, and count an appropriate amount of suspension; the optimal number of crypts is controlled at 5-15 per ul.
7. Take an appropriate volume of the suspension and add it to a 1.5ml centrifuge tube, centrifuge at 800rpm for 5 minutes at room temperature.
8. Discard the supernatant and resuspend with pre-cooled matrix gel (ensure thorough mixing).
9. Plate the cells, add 50ul of the resuspended liquid to each well of a 24-well plate (note: the matrix gel should be placed in the center of the well to avoid adhesion to the wall).
10. Place the plated wells in an incubator for 20-30 minutes.
11. Add 500ul of complete culture medium to each well, and change the medium every 2-3 days, passage the cells every 5-7 days.
Note: All operations should avoid contamination, and tissues should be kept on ice as much as possible.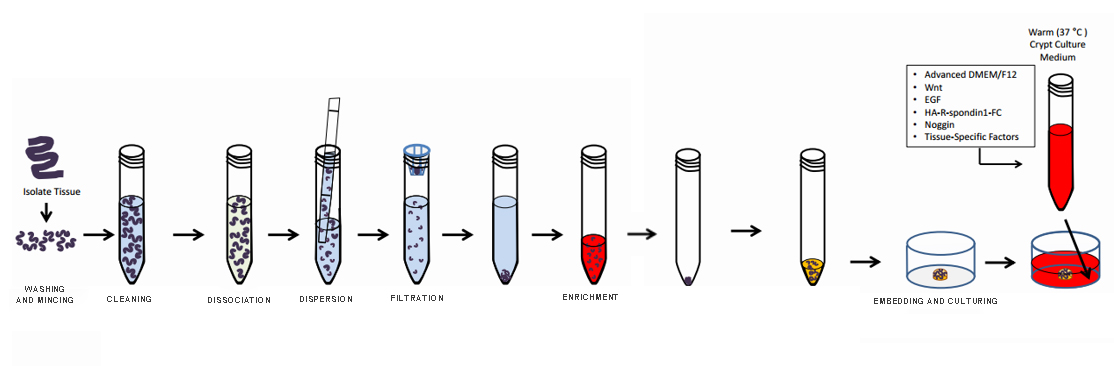
Small Intestine Organoid Culture Procedure Flowchart
1. Place the crypts to be passaged on ice for 5-10 minutes.
2. Carefully aspirate the supernatant and add 1ml of pre-cooled ADMEM. Use a large pipette tip to gently disrupt the matrix gel, then adjust to a 700ul volume and pipette up and down about 30 times to ensure thorough mixing. Avoid creating bubbles during this process. You can take an appropriate amount of the suspension for observation until no large clumps of crypts are visible.
3. Transfer the suspension to a 1.5ml centrifuge tube and centrifuge at 800rpm at room temperature for 5 minutes.
4. Follow the plating steps 8-11 as described in the crypt plating procedure.
Note: Theoretically, you can expand 3-5 times during each passage, but due to losses during the procedure, it is generally only possible to expand 3 times.|
Product Name |
Item NO. |
|
Human Colorectal cancer Organoid Culture Medium Kit |
Composition
|
Component |
Name |
Size |
Storage |
|
Component A |
Colorectal Cancer Organoid Buffer |
100ml |
4℃ |
|
Component B |
Colorectal Cancer Organoid Basal Medium |
100ml |
4℃ |
|
Component C |
organo Primary Enzymatic Digestion Liquid |
25ml |
4℃ |
|
Component D |
organo Digestion Liquid |
25ml |
4℃ |
|
Component E |
organo Primary Enzyme Termination Liquid |
100ml |
4℃ |
|
Component F |
Tissue Preservation Liquid |
100ml |
4℃ |
|
Component G |
organo Cryopreservation Liquid |
25ml |
4℃ |
Experimental Method
(1) Place the tissue in a tissue sampling vial containing special tissue preservation liquid F and keep it in a cooler box at 4°C. Take a photo and register the information.
(2) Sterilize the sampling vial, place the tissue in a culture dish, and wash it three times with colorectal cancer organoid buffer A before mincing it in the culture dish into tissue pieces approximately 1mm^3 in size.
(3) Digest the cancerous tissue with organo primary enzymatic digestion liquid C at 37°C with shaking for 10-20 minutes (observe the digestion process at any time), and then add three times the volume of organo primary enzyme termination liquid E to terminate the digestion.
(4) Filter through a sieve with a pore size of 100μm, take a small amount of the filtrate for observation under a microscope, where distinct tissue pieces should be visible. Collect the filtrate and after centrifugation at 300g for 3-5 minutes to remove the supernatant, resuspend and centrifuge again.
(5) Discard the supernatant, resuspend with an appropriate amount of matrix gel (abs9495) (for example, 30μl per well in a 24-well plate), then plate (operate at 4°C) and take a photo for tracking and positioning.
(6) Place the plated wells in a 37°C incubator for 10-15 minutes to allow the gel to set, then add culture medium B (returned to room temperature) for cultivation.
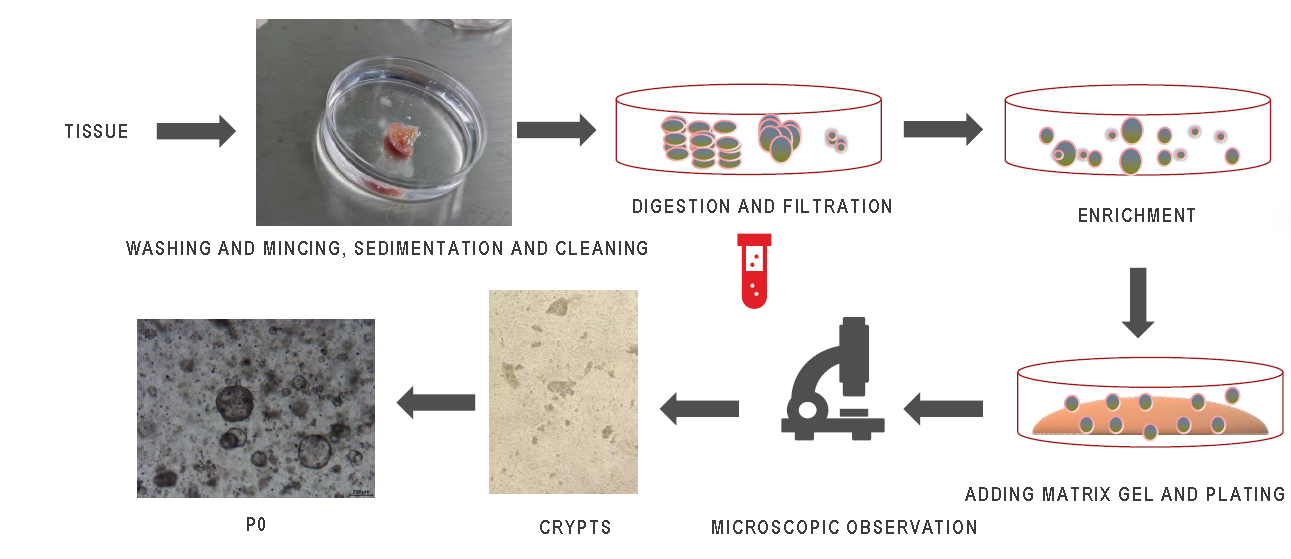
Organoid Culture Procedure (Matrix Gel Method)
(1) Aspirate the culture medium and add Organoid Buffer A at 4°C, let it sit for one minute.
(2) Gently pipette the matrix gel, collect it in a 15ml centrifuge tube, and let it stand at 4°C for 5 minutes.
(3) Centrifuge for 5 minutes, discard the liquid, resuspend with an appropriate amount of Organoid Buffer A, transfer to a 1.5ml centrifuge tube, and centrifuge at 300g for 5 minutes to discard the liquid, or (add an appropriate amount of Digestion Liquid D for 90-120 seconds to terminate, centrifuge for 5 minutes and discard the mixture).
(4) After collecting the organoids, add an appropriate amount of Organoid Cryopreservation Liquid G, and cryopreserve at a density of 500 per/ml (or proceed to step 5).
(5) After collecting the organoids, resuspend with matrix gel, and plate 30μl of matrix gel per well in a 24-well plate, let it sit in the incubator for 10 minutes before adding 500μl of Colorectal Cancer Organoid Culture Medium B.
(1) Take 10ml and place it in a 15ml centrifuge tube.
(2) Remove the frozen tumor organoid cells from the liquid nitrogen tank and quickly place them in a 37°C water bath to thaw.
(3) During the water bath thawing process, gently shake the frozen vial to ensure that the cryopreservation liquid is completely thawed within 1-2 minutes.
(4) Quickly transfer the thawed organoid cells to a 15ml sterile centrifuge tube, gently pipette 3 times, centrifuge at 300g for 3 minutes, then discard the supernatant and collect the organoid cell pellet. Resuspend with an appropriate amount of Colorectal Cancer Organoid Buffer A, transfer to a 1.5ml centrifuge tube, and centrifuge at 300g for 5 minutes.
(5) Resuspend with matrix gel, plate 20μl of matrix gel per well in a 24-well plate, let it sit in the incubator for 10 minutes before adding 500μl of Colorectal Cancer Organoid Culture Medium B.
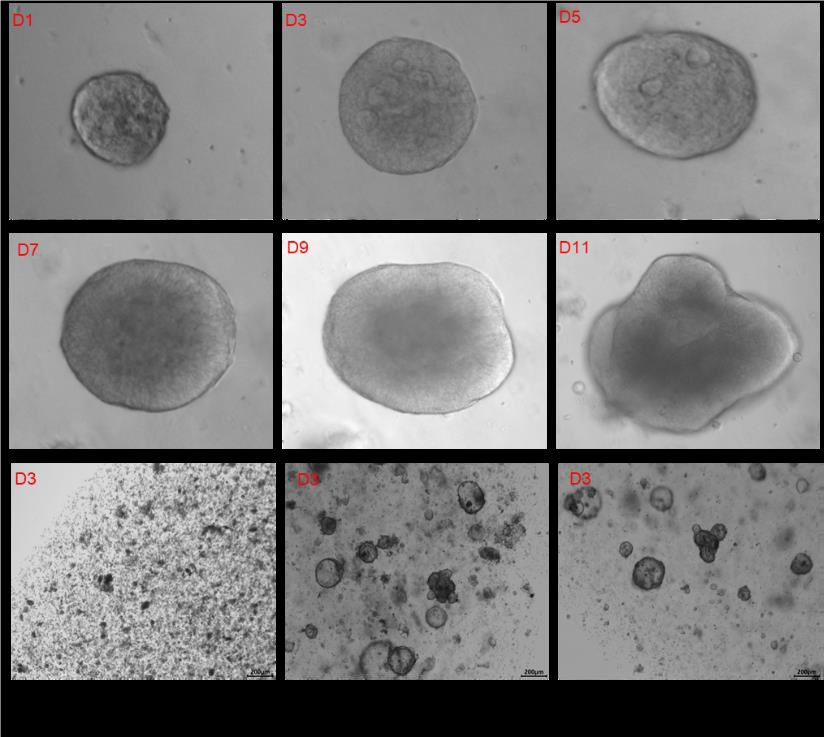
Culture Medium for Culturing Colorectal Cancer Organoids: Day 1 to Day 11 Culture Photos
|
Item NO. |
Product Name |
Size |
|
1kit |
||
|
1kit |
||
|
Mouse Lung Organoid Culture Medium Kit |
1kit |
|
|
Human Breast Cancer Organoid Culture Medium Kit |
1kit |
|
|
Human Endometrial carcinoma Organoid Culture Medium Kit |
1kit |
|
|
Human Colorectal cancer Organoid Culture Medium Kit |
1kit |
|
|
Human Lung Cancer Organoid Culture Medium Kit |
1kit |
|
|
1kit |
||
|
Human Pancreatic Cancer Organoid Culture Medium Kit |
1kit |
|
|
Human Ovarian Cancer Organoid Culture Medium Kit |
1kit |
|
|
Human Liver Organoid Culture Medium Kit |
1kit |
|
|
1kit |
||
|
Mouse Gastric Cancer Organoid Culture Medium Kit |
1kit |
|
|
1kit |
||
|
Mouse Breast Cancer Organoid Culture Medium Kit |
1kit |
|
|
Mouse Hepatocarcinoma Organoid Culture Medium Kit |
1kit |
|
|
1kit |
|
Item NO. |
Product Name |
Size |
|
Ready to use Matrigel |
100ml |
|
|
Standard OrganoGel with Phenol red |
1.5ml*8 |
|
|
Standard OrganoGel Phenol red free |
1.5ml*8 |
|
|
HC OrganoGel with Phenol red |
1.5ml*8 |
|
|
HC OrganoGel Phenol red free |
1.5ml*8 |
|
|
GFR OrganoGel with Phenol red |
1.5ml*8 |
|
|
GFR OrganoGel Phenol red free |
1.5ml*8 |
|
|
IPS-qualified OrganoGel Phenol red free |
1.5ml*4 |
|
|
HC&GFR OrganoGel with Phenol red |
1.5ml*8 |
|
|
HC&GFR OrganoGel Phenol red free |
1.5ml*8 |
参考文献:
[1] CORNING. Using Organoids for Disease Modeling. Nov. 11, 2019.
[2] Lee, J. et al. Nat Commun 11, 4283 (2020).
[3] SCIENCING. Types of Sea Sponges. 2019.
[4] Wilson, H. V. Development of sponges from dissociated tissue cells.
[5] Sato, T. et al. Nature 459, 262–265 (2009).
[6] Corrò C et al. Am J Physiol Cell Physiol. 2020 Jul 1;319(1):C151-C165.
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |
